Abstract
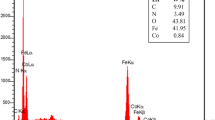
Nano-Al2O3/BF3/Fe3O4 was synthesized as an efficient and reusable catalyst. The synthesized magnetic catalyst has been characterized by various methods such as FT-IR, FESEM, TGA, TEM, VSM, XRF, XRD and BET. This catalyst does not need special precautions for preparation, handling or storage, and it can be stored at an ambient temperature for months without losing its catalytic activity. Five-substituted tetrahydropyridines and their derivatives have an interesting class of pharmaceutical activities. Thus, the nano-Al2O3/BF3/Fe3O4 catalyst was used to prepare five-substituted tetrahydropyridines by one-pot multicomponent reactions of aromatic aldehydes, anilines and β-keto-esters under solvent free conditions. The structure of products were studied by Fourier transform spectroscopy and nuclear magnetic resonance. The present protocol has notable advantages of easy purification, clean and convenient procedure and high yields for isolated products. In addition, this catalyst could be recycled several times without reduction in its admirable activity.
Graphical Abstract










Similar content being viewed by others
References
C. Srinivas, C.N.S.P. Kumar, B.C. Raju, V.J. Rao, V.G.M. Naidu, S. Ramakrishna, P.V. Diwan, Bioorg. Med. Chem. Lett. 19, 5915 (2009)
S. Petit, J.P. Nallet, M. Guillard, J. Dreux, R. Chermat, M. Poncelet, C. Bulach, P. Simon, C. Fontaine, M. Barthelmebs, J.L. Imbs, Eur. J. Med. Chem. 26, 19 (1991)
Y. Zhou, V.E. Gregor, B.K. Ayida, G.C. Winters, Z. Sun, D. Murphy, G. Haley, D. Bailey, J.M. Froelich, S. Fish, S.E. Webber, T. Hermann, D. Wall, Bioorg. Med. Chem. Lett. 17, 1206 (2007)
M. Misra, S.K. Pandey, V.P. Pandey, J. Pandey, R. Tripathi, R.P. Tripathi, Bioorgan. Med. Chem. 17, 625 (2009)
H. Bin, A.M. Crider, J.P. Stables, Eur. J. Med. Chem. 36, 265 (2001)
S.A. Khanum, V. Girish, S.S. Suparshwa, N.F. Khanum, Bioorg. Med. Chem. Lett. 19, 1887 (2009)
K.B. Domino, E.A. Anderson, N.L. Polissar, K.L. Posner, Anesth. Analg. 88, 1370 (1999)
A.T. Khan, T. Parvin, L.H. Choudhury, J. Org. Chem. 73, 8398 (2008)
P.A. Clarke, A.V. Zaytzev, A.C. Whitwood, Tetrahedron Lett. 48, 5209 (2007)
P.A. Clarke, A.V. Zaytsev, A.C. Whitwood, Synthesis 21, 3530 (2008)
A.T. Khan, M. Lal, M.M. Khan, Tetrahedron Lett. 51, 4419 (2010)
A.T. Khan, M.M. Khan, K.K. Bannuru, Tetrahedron Lett. 66, 7762 (2010)
H.-J. Wang, L.-P. Mo, Z.-H. Zhang, ACS. Comb. Sci. 13, 181 (2010)
S. Mishra, R. Ghosh, Tetrahedron Lett. 52, 2857 (2011)
R. Aeluri, M. Alla, V.R. Bommena, R. Murthy, N. Jain, Asian J. Org. Chem. 1, 71 (2012)
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, S.J. Shams-Najafi, Monatsh. Chem. 143, 939 (2012)
N. Hazeri, M.T. Maghsoodlou, S.M. Habibi-Khorassani, J. Aboonajmi, S.S. Sajadikhah, J. Chin. Chem. Soc. 60, 355 (2013)
J. Safaei-Ghomi, A. Ziarati, J. Iran. Chem. Soc. 10, 135 (2013)
G. Harichandran, S.D. Amalraj, P. Shanmugam, J. Heterocyclic Chem. 50, 539 (2013)
M. Lashkari, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, S.S. Sajadikhah, R. Doostmohamadi, Synth. Commun. 43, 635 (2013)
C. Mukhopadhyay, S. Rana, R.J. Butcher, A.M. Schmiedekamp, Tetrahedron Lett. 52, 5835 (2011)
M. Daraei, M.A. Zolfigol, F. Derakhshan-Panah, M. Shiri, H.G. Kruger, M. Mokhlesi, J. Iran. Chem. Soc. 12, 855 (2015)
H. Eshghi, A. Khojastehnezhad, F. Moeinpour, M. Bakavoli, S.M. Seyedi, M. Abbasi, RSC Adv. 4, 39782 (2014)
N. Salehi, B.F. Mirjalili, RSC Adv. 7, 30303 (2017)
B.F. Mirjalili, H. Jorsarraie, H.R. Akrami, Iran. J. Catal. 7, 201 (2017)
S. Azad, B.F. Mirjalili, Res. Chem. Intermed. 43, 1723 (2017)
B.F. Mirjalili, H. Bamoniri, S. Azad, J. Iran. Chem. Soc. 14, 47 (2017)
S. Azad, B.F. Mirjalili, RSC Adv. 6, 96928 (2016)
B.F. Mirjalili, H. Bamoniri, Z. Fazeli, Iran. J. Catal. 6, 253 (2016)
Acknowledgements
The Research Council of Yazd University is gratefully acknowledged for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Babaei, E., Mirjalili, B.B.F. One-pot synthesis of five substituted tetrahydropyridines using nano-Al2O3/BF3/Fe3O4 as a highly efficient nano-catalyst. Res Chem Intermed 44, 3493–3505 (2018). https://doi.org/10.1007/s11164-018-3320-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3320-5