Abstract
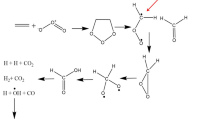
The cheletropic elimination process of N2 from (2,5-dihydro-1H-pyrrol-1-ium-1-ylidene) amide (C4H6N2) has been studied computationally using density functional theory, along with the M06-2X/aug-cc-pVTZ level of theory. The calculated energy profile has been supplemented with calculations of kinetic rate constants using transition state theory (TST) and statistical Rice–Ramsperger–Kassel–Marcus (RRKM) theory. This elimination process takes place spontaneously with an activation energy around 33 kJ/mol. Pressure dependence of the rate constants revealed that the TST approximation breaks down and fall-off expression is necessary for the kinetic modeling. At temperatures ranging from 240 to 360 K and atmospheric pressure, the unimolecular rate constant is evaluated from RRKM theory as \(k_{{(240 - 360\,{\text{K}})}}^{{1.0{\text{atm}}}} = 1.0249 \times 10^{12} \times {\text{e}}^{{ - \frac{{33.11\;{\text{kJ}}/{\text{mol}}}}{RT}}} \,{\text{s}}^{ - 1}\). Bonding changes along the reaction coordinate have been studied using bonding evolution theory. Electron localization function topological analysis reveals that the cheletropic elimination is characterized topologically by four successive structural stability domains (SSDs). Breaking of C–N bonds (Rx = 0.1992 amu1/2 Bohr) and the other selected points separating the SSDs along the reaction coordinate occur in the vicinity of the transition state.







Similar content being viewed by others
References
A.D. Becke, K.E. Edgecombe, A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 92, 5397–5403 (1990)
B. Silvi, A. Savin, Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371, 683–686 (1994)
S. Berski, J. Andrés, B. Silvi, L.R. Domingo, The joint use of catastrophe theory and electron localization function to characterize molecular mechanisms. A density functional study of the Diels-Alder reaction between ethylene and 1,3-butadiene. J. Phys. Chem. A 107, 6014–6024 (2003)
V. Polo, J. Andrés, A joint study based on the electron localization function and catastrophe theory of the chameleonic and centauric models for the Cope rearrangement of 1,5-hexadiene and its cyano derivatives. J. Comput. Chem. 26, 1427–1437 (2005)
V. Polo, J. Andrés, S. Berski, L.R. Domingo, B. Silvi, Understanding reaction mechanisms in organic chemistry from catastrophe theory applied to the electron localization function topology. J. Phys. Chem. A 112, 7128–7136 (2008)
S. Berski, J. Andrés, B. Silvi, L.R. Domingo, New findings on the Diels–Alder reactions. An analysis based on the bonding evolution theory. J. Phys. Chem. A 110, 13939–13947 (2006)
M. Ríos-Gutiérrez, L.R. Domingo, P. Pérez, Understanding the high reactivity of carbonyl compounds towards nucleophilic carbenoid intermediates generated from carbene isocyanides. RSC Adv. 5, 84797–84809 (2015)
L.R. Domingo, P. Pérez, J.A. Sáez, Understanding the regioselectivity in hetero Dielse–Alder reactions. An ELF analysis of the reaction between nitrosoethylene and 1-vinylpyrrolidine. Tetrahedron 69, 107–114 (2013)
L.R. Domingo, P. Pérez, J.A. Sáez, Understanding C–C bond formation in polar reactions. An ELF analysis of the Friedel–Crafts reaction between indoles and nitroolefins. RSC Adv. 3, 7520–7528 (2013)
L.R. Domingo, M.J. Aurella, P. Pérez, The mechanism of ionic Diels–Alder reactions. A DFT study of the oxa-Povarov reaction. RSC Adv. 4, 16567–16577 (2014)
L.R. Domingo, A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 4, 32415–32428 (2014)
L.R. Domingo, J.A. Sáez, Understanding the selectivity in the formation of δ-lactams vs. β-lactams in the Staudinger reactions of chloro-cyan-ketene with unsaturated imines. A DFT study. RSC Adv. 4, 58559–58566 (2014)
L.R. Domingo, M. Ríos-Gutiérrez, P. Pérez, Unravelling the mechanism of the ketene-imine Staudinger reaction. An ELF quantum topological analysis. RSC Adv. 5, 37119–37129 (2015)
L.R. Domingo, M.J. Aurella, P. Pérez, Understanding the polar mechanism of the ene reaction. A DFT study. Org. Biomol. Chem. 12, 7581–7590 (2014)
L.R. Domingo, M. Ríos-Gutiérrez, P. Pérez, A DFT study of the ionic [2 + 2] cycloaddition reactions of keteniminium cations with terminal acetylenes. Tetrahedron 71, 2421–2427 (2015)
P. Pérez, L.R. Domingo, A DFT study of inter- and intra-molecular aryne ene reactions. Eur. J. Org. Chem. 2015, 2826–2834 (2015)
A.K. Nacereddine, C. Sobhi, A. Djerourou, M. Ríos-Gutiérrez, L.R. Domingoc, Non-classical CH/O hydrogen-bond determining the regio- and stereoselectivity in the [3 + 2] cycloaddition reaction of (Z)-C-phenyl-Nmethylnitrone with dimethyl 2-benzylidenecyclopropane-1,1-dicarboxylate. A topological electron-density study. RSC Adv. 5, 99299–99311 (2015)
V. Polo, L.R. Domingo, J. Andrés, Better understanding of the ring-cleavage process of cyanocyclopropyl anionic derivatives. A theoretical study based on the electron localization function. J. Org. Chem. 71, 754–762 (2006)
L.R. Domingo, E. Chamorro, P. Pérez, Understanding the mechanism of non-polar Diels–Alder reactions. A comparative ELF analysis of concerted and stepwise diradical mechanisms. Org. Biomol. Chem. 8, 5495–5504 (2010)
L.R. Domingo, Why Diels–Alder reactions are non-concerted processes. J. Chil. Chem. Soc. 59, 2615–2618 (2014)
E.V. Anslyn, D.A. Dougherty, Modern Physical Organic Chemistry (University Science Books, Sausalito, 2006)
J.P. Buxton, C.J.S.M. Simpson, Thermal decarbonylations of unsaturated cyclic ketones: kinetics and dynamics. Chem. Phys. 105, 307–316 (1986)
D.M. Birney, S. Ham, G.R. Unruh, Pericyclic and pseudopericyclic thermal cheletropic decarbonylations: When can a pericyclic reaction have a planar, pseudopericyclic transition state? J. Am. Chem. Soc. 119, 4509–4517 (1997)
N.S. Isaacs, A.A.R. Laila, Rates of addition of sulphur dioxide to some 1,3-dienes. Tetrahedron Lett. 17, 715–716 (1976)
D. Suárez, E. Iglesias, T.L. Sordo, J.A. Sordo, Mechanism of cheletropic reactions of 1,3-dienes with sulfur dioxide. J. Phys. Org. Chem. 9, 17–20 (1996)
D.L. Lemal, S.D. McGregor, Dienes from 3-pyrrolines. A stereospecific deamination. J. Am. Chem. Soc. 88, 1335–1336 (1966)
H. Eyring, The activated complex and the absolute rate of chemical reactions. Chem. Rev. 17, 65–77 (1935)
D.G. Truhlar, B.C. Garrett, S.J. Klippenstein, Current status of transition-state theory. J. Phys. Chem. 100, 12771–12800 (1996)
A. Fernandez-Ramos, B.A. Ellingson, B.C. Garrett, D.G. Truhlar, Variational transition state theory with multidimensional tunneling. Rev. Comput. Chem. 23, 125–232 (2007)
W. Forst, Unimolecular rate theory test in thermal reactions. J. Phys. Chem. 76, 342–348 (1972)
W. Forst, Unimolecular Reactions. A Concise Introduction (Cambridge University Press, Cambridge, 2003)
P.J. Robinson, K.A. Holbrook, Unimolecular Reactions (Wiley, New York, 1972)
K.A. Holbrook, M.J. Pilling, S.H. Robertson, Unimolecular Reactions, 2nd edn. (Wiley, Chichester, 1996)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery-Jr., J.E. Peralta, F. Ogliaro, M. Bearpar, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, O. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian 09, Revision A.02-SMP., in, Gaussian, Inc., Wallingford CT (2009)
X. Li, M.J. Frisch, Energy-represented DIIS within a hybrid geometry optimization method. J. Chem. Theory Comput. 2, 835–839 (2006)
Y. Zhao, D.G. Truhlar, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06 functionals and twelve other functionals. Theor. Chem. Acc. 120, 215–241 (2008)
Y. Zhao, D.G. Truhlar, Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167 (2008)
T.H. Dunning Jr., Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007–1023 (1989)
H.P. Hratchian, H.B. Schlegel, Accurate reaction paths using a Hessian based predictor-corrector integrator. J. Chem. Phys. 120, 9918–9924 (2004)
H.P. Hratchian, H.B. Schlegel, Theory and Applications of Computational Chemistry: The First 40 Years (Elsevier, Amsterdam, 2005)
H.P. Hratchian, H.B. Schlegel, Using Hessian updating to increase the efficiency of a Hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 1, 61–69 (2005)
J.I. Steinfeld, J.S. Francisco, W.L. Hase, Chemical Kinetics and Dynamics, 2nd edn. (Prentice-Hall Inc, Upper Saddle River, 1999)
E. Wigner, Über das überschreiten von potentialschwellen bei chemischen reaktionen. Z. Phys. Chem. Abt. B 19, 203–216 (1932)
C. Eckart, The penetration of a potential barrier by electrons. Phys. Rev. 35, 1303–1309 (1930)
F.M. Mourits, F.H.A. Rummens, A critical evaluation of Lennard-Jones and Stockmayer potential parameters and of some correlation methods. Can. J. Chem. 55, 3007–3020 (1977)
S. Canneaux, F. Bohr, E. Henon, KiSThelP: kinetic and statistical thermodynamical package (2014)
Computational chemistry comparison and benchmark dataBase, precomputed vibrational scaling factors. http://cccbdb.nist.gov/vibscalejust.asp
X. Krokidis, S. Noury, B. Silvi, Characterization of elementary chemical processes by catastrophe theory. J. Phys. Chem. A 101, 7277–7282 (1997)
L.R. Domingo, P. Pérez, A quantum chemical topological analysis of the C–C bond formation in organic reactions involving cationic species. Phys. Chem. Chem. Phys. 16, 14108–14115 (2014)
A. Savin, B. Silvi, F. Colonna, Topological analysis of the electron localization function applied to delocalized bonds. Can. J. Chem. 74, 1088–1096 (1996)
S. Noury, X. Krokidis, F. Fuster, B. Silvi, TopMod package (1997)
F.L. Hirshfeld, Bonded-atom fragments for describing molecular charge densities. Theor. Chem. Acc. 44, 129138 (1977)
Acknowledgments
The authors thank anonymous referees for highly relevant comments. E. Zahedi expresses his gratitude to the Islamic Azad University, Shahrood Branch.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zahedi, E., Mozaffari, M., Shahsavar, F. et al. Understanding the kinetics and mechanism of thermal cheletropic elimination of N2 from (2,5-dihydro-1H-pyrrol-1-ium-1-ylidene) amide using RRKM and ELF theories. Res Chem Intermed 43, 1575–1590 (2017). https://doi.org/10.1007/s11164-016-2716-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2716-3