Abstract
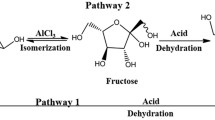
We synthesized 5-hydroxymethylfurfural (HMF) from carbohydrates using metal chloride catalysts. A 33.2 % yield of HMF was obtained from raw Dioscorea composita biomass with high starch by using a catalyst system composed of CrCl3·6H2O and LaCl3·6H2O at 120 °C for 4 h in N,N-dimethylacetamide containing lithium chloride. The catalyst system is also cost-effective for the conversion of soluble starch into HMF. In addition, levulinic acid was not formed in the reactions.



Similar content being viewed by others
References
A.E. Farrell, R.J. Pelvin, B.T. Turner, A.D. Jones, M. O’hare, D.M. Kammen, Science 311, 506 (2006)
P. Szuromi, B. Jasny, D. Clery, J. Austin, B. Hanson, Science 315, 781 (2007)
M. Bicker, J. Hirth, H. Vogel, Green Chem. 5, 280 (2003)
D.A. Simonetti, J.A. Dumesic, ChemSusChem 1, 725 (2008)
J.N. Chheda, G.W. Hubera, J.A. Dumesic, Angew. Chem. Int. Ed. 46, 7164 (2007)
R.-J. van Putten, J.C. van der Waal, E. de Jong, C.B. Rasrendra, H.J. Heeres, J.G. de Vries, Chem. Rev. 2013(113), 1499 (2013)
Y. Roman-Leshkov, C.J. Barrett, Z.Y. Liu, J.A. Dumesic, Nature 2007(447), 982 (2007)
M. Mascal, E.B. Nikitin, Angew. Chem. Int. Ed. 47, 7924 (2008)
T. Stahlberg, W.J. Fu, J.M. Woodley, A. Riisager, ChemSusChem 4, 451 (2011)
T. Buntara, S. Noel, P.H. Phua, I. Melian-Cabrera, J.G. de Vries, H.J. Heeres, Angew. Chem. Int. Ed. 50, 7083 (2011)
C.H. Zhou, X. Xia, C.X. Lin, D.S. Tong, J. Beltramini, Chem. Soc. Rev. 40, 5588 (2011)
J.N. Chheda, Y. Roman-Leshkov, J.A. Dumesic, Green Chem. 9, 342 (2007)
Y. Yang, X. Xiang, D.M. Tong, C.W. Hu, M.M. Abu-Omar, Bioresource Technol. 116, 302 (2012)
Y. Yang, C.W. Hu, M.M. Abu-Omar, Green Chem. 14, 509 (2012)
S.Q. Hu, Z.F. Zhang, J.L. Song, Y.X. Zhou, B.X. Han, Green Chem. 11, 1746 (2009)
J.A. Chun, J.W. Lee, Y.B. Yi, S.S. Hong, C.H. Chung, Starch/Stärke 62, 326 (2010)
Y.B. Yi, M.G. Ha, J.W. Lee, C.H. Chung, Chem. Eng. J. 180, 370 (2012)
T.F. Wang, Y.J. Pagan-Torres, E.J. Combs, J.A. Dumesic, B.H. Shanks, Top. Catal. 55, 657 (2012)
X.L. Tong, L.H. Yu, G.X. Nie, Z.M. Li, J.B. Liu, S. Xue, Environ. Prog. Sustain. 34, 1136 (2015)
H.B. Zhao, J.E. Holladay, H. Brown, Z.C. Zhang, Science 316, 1597 (2007)
J.B. Binder, R.T. Raines, J. Am. Chem. Soc. 131, 1979 (2009)
C. Wang, L. Fu, X.L. Tong, Q.W. Yang, W.Q. Zhang, Carbohydr. Res. 347, 182 (2012)
B. Kim, J. Jeong, D. Lee, S. Kim, H.J. Yoon, Y.S. Lee, J.K. Cho, Green Chem. 13, 1503 (2011)
K.I. Seri, Y. Inoue, H. Ishida, Chem. Lett. 1, 22 (2000)
T. Heinze, P. Talaba, U. Heinze, Carbohydr. Polym. 42, 411 (2000)
M. Terbojevich, A. Cosani, G. Conio, A. Ciferri, Macromolecules 18, 640 (1985)
C.L. McCormick, P.A. Callais, Macromolecules 18, 2394 (1985)
J.W. Lee, M.G. Ha, Y.B. Yi, C.H. Chunget, Carbohydr. Res. 346, 177 (2011)
V. Bellon-Maurel, C. Vallat, D. Goffinet, Appl. Spectrosc. 49, 556 (1995)
Y.B. Yi, M.G. Ha, J.W. Lee, C.H. Chung, Chem. Eng. J. 180, 370 (2012)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, F., Shi, G., Wang, G. et al. Catalytic conversion of raw Dioscorea composita biomass to 5-hydroxymethylfurfural using a combination of metal chlorides in N,N-dimethylacetamide solvent containing lithium chloride. Res Chem Intermed 42, 6757–6767 (2016). https://doi.org/10.1007/s11164-016-2496-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2496-9