Abstract
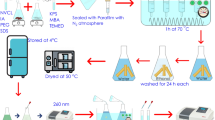
In this study, N-vinylcaprolactam, metacrylic acid sodium salt and itaconic acid sodium salt-based copolymeric and terpolymeric microgels were synthesized by precipitation polymerization method with 2,2′-azobis(2-methylpropioamidine) dihydrochloride as initiator. Then these microgels were characterized by SEM technique, cloud points and colloidal properties determinations. Volume phase transitions of copolymeric and terpolymeric N-vinylcaprolactam-based microgels are determined at an interval of 32–37 °C. Rhodamine B (model drug) and Nadalol (beta-blocker drug) were used to investigate the drug loading and release behavior of microgels. It is concluded that model drug loading capacity and release amount changed with the presence and amount of itaconic acid sodium salt in the microgel structure. In addition, the maximum drug release amount of microgels was found to be 58 and 55 % for Rhodamine B and Nadolol, respectively. As a result, we can say that the microgels obtained in this study are suitable for drug delivery applications.










Similar content being viewed by others
References
S. Chaterji, I.K. Kwon, K. Park, Prog. Polym. Sci. 32, 1083–1122 (2007)
J. Kopecek, Nature 417, 388–391 (2002)
S. Zhou, B. Chu, J. Phys. Chem. B 102, 1364–1371 (1998)
I. Varga, T. Gilanyi, R. Meszaros, G. Filipcsei, M. Zrinyi, J. Phys. Chem. B 105, 9071–9076 (2001)
R. Pelton, Adv. Colloid Interface Sci. 85, 1–33 (2000)
B.R. Saunders, N. Laajam, E. Daly, S. Teow, X. Hu, R. Stepto, Adv. Colloid Interface Sci. 147–148, 251–262 (2009)
B.R. Saunders, B. Vincent, Adv. Colloid Interface Sci. 80, 1–25 (1999)
A. Imaz, J. Forcada, J. Polym. Sci., Part A: Polym. Chem. 46, 2766–2772 (2008)
S. Berger, R. Singh, J.D. Sudha, H.J. Adler, A. Pich, Polymer 51, 3829–3835 (2010)
X.J. Ju, L. Liu, R. Xie, C.H. Niu, L.Y. Chu, Polymer 50, 922–929 (2009)
Y. Wang, J. Nie, B. Chang, Y. Sun, W. Yang, Biomacromolecules 14, 3034–3046 (2013)
V.C. Lopez, S.L. Raghavan, M.J. Snowden, React. Funct. Polym. 58, 175–185 (2004)
V.C. Lopez, J. Hadgraft, M.J. Snowden, Int. J. Pharm. 292, 137–147 (2005)
D. Crespy, R.M. Rossi, Polym. Int. 56, 1461–1468 (2007)
N.I. Shtanko, W. Lequieu, E.J. Goethals, F.E. Prez, Polym. Int. 52, 1605–1610 (2003)
S. Shah, A. Pal, R. Gude, S. Devi, Eur. Polym. J. 46, 958–967 (2010)
N.S. Rejinold, M. Muthunarayanan, V.V. Divyarani, P.R. Sreerekha, K.P. Chennazhi, S.V. Nair, J. Colloid Interface Sci. 360, 39–51 (2011)
V. Boyko, A. Pich, Y. Lu, S. Richter, K.F. Arndt, H.J.P. Adler, Polymer 44, 7821–7827 (2003)
J.B. Thorne, G.J. Vine, M.J. Snowden, Colloid Polym. Sci. 289, 625–646 (2011)
C. Ramkissoon-Ganorkar, F. Liu, M. Baudys, S.W. Kim, J. Controlled Release 59, 287–298 (1999)
Y. Zhang, W. Zhu, B. Wang, J. Ding, J Control Release 105, 260–268 (2005)
F. Meeussen, E. Nies, H. Berghmans, S. Verbrugghe, E. Guethals, F. Du Prez, Polymer 41, 8597–8602 (2000)
Z. Nart, N. Kayaman Apohan, J. Polym. Res. 18, 869–874 (2011)
J.P.K. Tan, C.H. Goh, K.C. Tam, Eur. J. Pharm. Sci. 32, 340–348 (2007)
S. Lou, S. Gao, W. Wang, M. Zhang, Q. Zhang, C. Wang, C. Li, D. Kong, J. Appl. Polym. Sci. 131, 1–7 (2014). doi:10.1002/app.41146
H. Vihola, A. Laukkanen, J. Hirvonen, H. Tenhu, Eur. J. Pharm. Sci. 16, 69–74 (2002)
Acknowledgments
This work is a part of the Ph.D. thesis titled “Usage of Polymeric Hydrogels and Microgels in Drug Release Applications”, prepared at Istanbul University, Institute of Science, and supported by the Research Fund of the Istanbul University, Project Number: 29693.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özkahraman, B., Acar, I., Gök, M.K. et al. N-vinylcaprolactam-based microgels: synthesis, characterization and drug release applications. Res Chem Intermed 42, 6013–6024 (2016). https://doi.org/10.1007/s11164-016-2422-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2422-1