Abstract
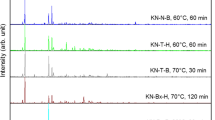
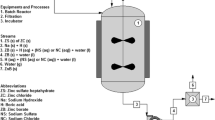
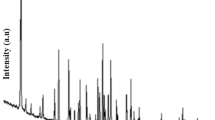
In this study, potassium pentaborate (santite: KB5O8·4H2O), with a powder diffraction number of 01-072-1688 was synthesized from potassium carbonate (K2CO3), boric acid (H3BO3) and boron oxide (B2O3) with reaction efficiencies between 84.88 and 95.11 %, through a hydrothermal route. Reaction temperatures and times were varied between 60–90 °C and 15–120 min. Synthesized minerals were characterized by X-ray diffraction, Fourier transform infrared and Raman spectroscopies, while surface morphologies were determined by scanning electron microscopy. Thermal dehydration behavior of santite was determined by thermal gravimetry and differential thermal analysis. From the results, santite lost its crystal water via a two-step process between 50 and 450 °C. Activation energies (E a) were calculated by using four non-isothermal kinetic methods and found to be 110.12 and 202.43 kJ/mol by the Coats–Redfern method, 107.77 and 304.18 kJ/mol by the Doyle method, 158.82 and 154.50 kJ/mol by the Kissinger–Akahira–Sunose method, and 158.07 and 156.76 kJ/mol by the Ozawa–Flynn–Wall method for the steps 1 and 2, respectively.










Similar content being viewed by others
References
A.S. Kipcak, E. Moroydor Derun, J. Piskin, J. Chem. 2013, 329238 (2013)
E.M. Derun, A.S. Kipcak, J. Radioanal. Nucl. Chem. 292, 871 (2012)
L.D. Pye, V.D. Frechette, N.J. Kreidl, Borate Glasses: Structure, Properties, Applications, 1st edn. (Plenum, New York, 1978)
G. Yang, Z. Li, Y. Zhang, Chem. Eng. Process. 44, 1216 (2005)
K. Thamizharasan, S.X. Jesu Raja, F.P. Xavier, P. Sagayaraj, J. Cryst. Growth 218, 323 (2000)
S. Merlino, F. Sartori, Contr. Mineral. Petrol. 27, 159 (1970)
W.A. Gale, Method of preparing potassium pentaborate. Patented Oct. 5, 1937 (No. 45, 57)
W.M. MacDonald, A.-C. Anderson, J. Schoreder, Phys. Rev. B 32, 1208 (1985)
Y.S.M. Alajerami, S. Hashim, W.M.S. Wan Hassan, A.T. Ramli, J. Mol. Struct. 1026, 159 (2012)
P.R. Binu, C.M. Joseph, C.S. Menon, K. Shreekrishnakumar, Mater. Lett. 52, 323 (2002)
S. Hashim, Y.S.M. Alajerami, A.T. Ramli, S.K. Ghoshal, M.A. Saleh, A.B. AbdulKadir, M.I. Saripan, K. Alzimami, D.A. Bradley, M.H.A. Mhareb, Appl. Radiat. Isotopes 91, 126 (2014)
O. Sahin, E.S. Cennetkusu, H. Dolas, M. Ozdemir, Thermochim. Acta 440, 7 (2005)
E.L. Belokoneva, S.Y. Stefanovich, O.V. Dimitrova, J. Solid State Chem. 195, 79 (2012)
G. Wang, Y.G. Sun, S. Zheng, G. Yang, Z. Anorg, Allg. Chem. 632, 1586 (2006)
S.A. Rajasekar, K. Thamizharasan, A. Joseph Arul Pragasam, J. Packiam Julius, P. Sagayaraj, J. Cryst. Growth 247, 199 (2003)
H. Gurbuz, G. Badem, A.N. Bulutcu, J. Cryst. Growth 283, 222 (2005)
M. Tunc, H. Ersahan, S. Yapici, S. Colak, J. Therm. Anal. 48, 403 (1997)
A.K. Galwey, Thermochim. Acta 355, 181 (2000)
F. Sevim, F. Demir, M. Bilen, H. Okur, Korean J. Chem. Eng. 23, 736 (2006)
A. Kantürk, M. Sarı, S. Piskin, Korean J. Chem. Eng. 6, 1331 (2008)
E.M. Derun, F.T. Senberber, Sci. World J. 2014, 985185 (2014)
E.M. Derun, A.S. Kipcak, F.T. Senberber, M.S. Yilmaz, Res. Chem. Intermed. 41, 853 (2015)
A.K. Figen, M.S. Yılmaz, S. Piskin, Mater. Charact. 6(61), 640–647 (2010)
A.S. Kipcak, F.T. Senberber, E.M. Derun, N. Tugrul, S. Piskin, Res. Chem. Intermed. 41, 9129 (2015)
M. Touboul, E. Betourne, Solid State Ionics 84, 189–197 (1996)
Y. Erdogan, A. Zeybek, A. Sahin, A. Demirbas, Thermochim. Acta 326, 99 (1999)
M.S. Yılmaz, S. Piskin, J. Chem. Soc. Pak. 34(3), 526 (2012)
J. Yongzhong, G. Shiyang, X. Shuping, L. Lun, Spectrochim. Acta A 56, 1291 (2000)
L. Jun, X. Shuping, G. Shiyang, Spectrochim. Acta 51A, 519 (1995)
A.S. Kipcak, D.Y. Baysoy, E.M. Derun, S. Piskin, Adv. Mater. Sci. Eng. 2013, 747383 (2013)
A.S. Kipcak, E.M. Derun, S. Piskin, Turk. J. Chem. 38, 792 (2014)
A.S. Kipcak, M. Yildirim, S.A. Yuksel, E.M. Derun, S. Piskin, Adv. Mater. Sci. Eng. 2014, 819745 (2014)
H.S. Fogler, Element of Chemical Reaction Engineering, 3rd edn. (Prentice-Hall, New Jersey, 1999)
M.S. Yilmaz, A.K. Figen, S. Piskin, Res. Chem. Intermed. 41, 1893 (2015)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asensio, M.O., Yildirim, M., Senberber, F.T. et al. Thermal dehydration kinetics and characterization of synthesized potassium borates. Res Chem Intermed 42, 4859–4878 (2016). https://doi.org/10.1007/s11164-015-2326-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2326-5