Abstract
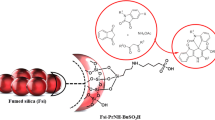
Silica-supported molybdic acid (SSMA) as a novel and highly efficient catalyst was prepared and characterized by X-ray fluorescence, X-ray diffraction, and Fourier transform infrared spectroscopy. Catalytic activity of as-prepared SSMA was then investigated for synthesis of novel azo coumarin dyes. This convenient and efficient protocol provides better alternatives to existing methods because the reactions proceeded quickly under clean conditions with high yields.
Graphical Abstract






Similar content being viewed by others
References
A. Corma, H. Garcia, Adv. Synth. Catal. 348, 1391–1412 (2006)
M.A. Satam, R.K. Raut, N. Sekar, Dyes Pigm. 96, 92–103 (2013)
A.D. Towns, Dyes Pigm. 42, 3–28 (1999)
K.L. Georgiadou, E.G. Tsatsaroni, Dyes Pigm. 50, 93–97 (2001)
A.S. Abd-El-Aziz, T.H. Afifi, Dyes Pigm. 70, 8–17 (2006)
R. Cristiana, A.M. Hossu, I. Ionita, Dyes Pigm. 71, 123–129 (2006)
H. Valizadeh, A. Shomali, S. Nourshargh, Dyes Pigm. 113, 522–528 (2015)
H. Khanmohammadi, K. Rezaeian, M.M. Amini, N.S. Weng, Dyes Pigm. 98, 557–564 (2013)
J. Koh, A.J. Greaves, Dyes Pigm. 50, 117–126 (2001)
H.S. Bhatti, S. Seshadri, Color. Technol. 120, 151–155 (2004)
E. Abdel-latif, M.A. Metwally, Mon. Chem. 138, 771–776 (2007)
J. Merrington, M. James, M. Bradley, Chem. Commun. 2, 140–141 (2002)
A.R. Hajipour, A. Zarei, A.E. Ruoho, Synth. Commun. 36, 1039–1050 (2006)
S.A. Hameed, Jord. J. Chem. 2, 133–144 (2007)
H. Valizadeh, M. Amiri, A. Shomali, F. Hosseinzadeh, J. Iran. Chem. Soc. 8, 495–501 (2011)
H. Valizadeh, M. Amiri, F. Hosseinzadeh, Dyes Pigm. 92, 1308–1313 (2012)
S. Khodabakhshi, B. Karami, Catal. Sci. Technol. 2, 1940–1944 (2012)
M.L. Kantam, M. Roy, S. Roya, B. Sreedhara, R.L. De, Catal. Commun. 9, 2226–2230 (2008)
A.D. Murkute, J.E. Jackson, D.J. Miller, J. Catal. 278, 189–199 (2011)
A. Cornelis, P. Laszlo, Synthesis 909–918 (1985)
B. Karami, M. Kiani, Catal. Commun. 14, 62–67 (2011)
B. Karami, Z. Haghighijou, M. Farahi, S. Khodabakhshi, Phosphorus Sulfur Silicon Relat. Elem. 187, 754–761 (2012)
B. Karami, S. Khodabakhshi, M. Jamshidi, J. Chin. Chem. Soc. 60, 1103–1106 (2013)
S. Khodabakhshi, B. Karami, K. Eskandari, S.J. Hoseini, A. Rashidi, RSC Adv. 4, 17891–17895 (2014)
H. Karade, M. Sathe, M.P. Kaushik, Catal. Commun. 8, 741–746 (2007)
T. Brezesinski, J. Wang, S.H. Tolbert, B. Dunn, Nat. Mater. 9, 146–151 (2010)
S. Khodabakhshi, B. Karami, K. Eskandari, C. R. Chim. 17, 35–40 (2014)
B. Karami, K. Eskandari, S. Khodabakhshi, J. Iran. Chem. Soc. 11, 631–637 (2014)
B. Karami, S. Khodabakhshi, Z. Haghighijou, Chem. Pap. 66, 684–690 (2012)
K. Eskandari, B. Karami, S. Khodabakhshi, Catal. Commun. 54, 124–130 (2014)
B. Karami, S. Khodabakhshi, F. Hashemi, Tetrahedron Lett. 54, 3583–3585 (2013)
B.B.F. Mirjalili, A. Bamoniri, A. Akbari, Curr. Chem. Lett. 1, 109–114 (2012)
A. Zarei, A.R. Hajipour, L. Khazdooz, B.B.F. Mirjalili, A.N. Chermahini, Dyes Pigm. 81, 240–244 (2009)
Acknowledgments
The authors are grateful for the support from the Yasouj University (Iran).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karami, B., Kiani, M. Novel silica-supported molybdic acid (SSMA): an efficient catalyst for the synthesis of new azo dyes. Res Chem Intermed 42, 3373–3383 (2016). https://doi.org/10.1007/s11164-015-2218-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2218-8