Abstract
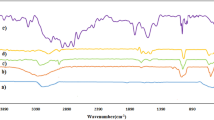
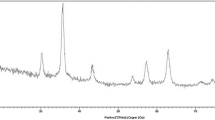
Pyridine-4-carboxylic acid (PYCA) functionalized Fe3O4 nanoparticles as an organic–inorganic hybrid heterogeneous catalyst was fabricated and characterized by FT-IR, XRD, TGA, TEM, SEM, and VSM techniques. The catalytic activity of the magnetic catalyst was probed through one-pot synthesis of pyrano[3,2-b]pyranone derivatives from three component reactions of aromatic aldehydes, kojic acid, and ethyl cyanoacetate under solvent-free conditions. Some advantages of this protocol are its environmentally benign method, simple procedure, high yields, and short reaction time. The catalyst was readily separated using an external magnet and reusable without significant loss of its catalytic efficiency.












Similar content being viewed by others
References
A. Tiwari, A.K. Mishra, H. Kobayashi, A.P.F. Turner, Intelligent Nanomaterials: Processes, Properties, and Applications (John Wiley & Sons, Inc., New Jersey, 2012)
B. Hu, J. Pan, H.L. Yu, J.W. Liu, J.H. Xu, Process Biochem. 44, 1019–1024 (2009)
K.J. Klabunde, R. Mulukutla, Chemical and Catalytic Aspects of Nanocrystals. Nanoscale Materials in Chemistry (Wiley Interscience, NewYork, 2001)
C.N.R. Rao, A. Müller, K. Anthony, Nanomaterials Chemistry: Recent Developments and New Directions (John Wiley & Sons, Inc., New Jersey, 2007)
A.H. Lu, E.L. Salabas, F. Schuth, Angew. Chem. Int. Ed. 46, 1222–1244 (2007)
C.S. Gill, B.A. Price, C.W. Jones, J. Catal. 251, 145–152 (2007)
A. Taher, J.B. Kim, J.Y. Jung, W.S. Ahn, M.J. Jin, Synlett 15, 2477–2482 (2009)
O.C. Dalaigh, A.S. Corr, Y. Gunko, J.S. Connon, Angew. Chem. Int. Ed. 46, 4329–4332 (2007)
Y. Zhang, Y. Zhao, C. Xia, J. Mol. Catal. A: Chem. 306, 107–112 (2009)
D. Wang, D. Astruc, Chem. Rev. 114, 6949–6985 (2014)
Q.M. Kainz, O. Reiser, Acc. Chem. Res. 47, 667–677 (2014)
R.B. Nasir Baig, M.N. Nadagouda, R.S. Varma, Coord. Chem. Rev. 287, 137–156 (2014)
J. Zhu, H. Bienayme, Multicomponent Reactions (Wiley-VCH, Weinheim, 2005)
A. Dömling, Chem. Rev. 106, 17–89 (2006)
S. Jimenez-Alonso, H. Chavez, A. Estevez-Braan, A. Ravelo, G. Feresin, A. Tapia, Tetrahedron 64, 8938–8942 (2008)
D.F. Tejedor, G. Tellado, Chem. Soc. Rev. 36, 484–491 (2007)
A. Nefzi, J.M. Ostresh, R.A. Houghten, Chem. Rev. 97, 449–472 (1997)
L.A. Thompson, Curr. Opin. Chem. Biol. 4, 324–337 (2000)
A. Dömling, Curr. Opin. Chem. Biol. 6, 306–313 (2002)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517–520 (1993)
F.M. Uckun, C. Mao, A.O. Vassilev, H. Huang, S.T. Jan, Bioorg. Med. Chem. Lett. 10, 541–545 (2000)
R. Gonzalez, N. Martin, C. Seoane, J.L. Marco, A. Albert, F.H. Cano, Tetrahedron Lett. 33, 3809–3812 (1992)
D. Armesto, W.M. Horspool, N. Martin, A. Ramos, C. Seaone, J. Org. Chem. 54, 3069–3072 (1989)
S. Asghari, M. Ahmadipour, Acta. Chem. Slov. 57, 953–956 (2010)
M.B. Miya, Y.J. Prakash Raob, G.L.D. Krupadanam, Heterocycl. Lett. 2, 214–217 (2012)
S. Paul, P. Bhattacharyya, A.R. Das, Tetrahedron Lett. 52, 4636–4641 (2011)
L.M. Wang, N. Jiao, J. Qiu, J.J. Yu, J.Q. Liu, F.L. Guo, Y. Liu, Tetrahedron 66, 339–343 (2010)
H.J. Wang, J. Lu, Z.H. Zhang, Monatsh. Chem. 141, 1107–1112 (2010)
M. Saeedi, M.M. Heravi, Y.S. Beheshtiha, H.A. Oskooie, Tetrahedron 66, 5345–5348 (2010)
U.R. Pratap, D.V. Jawale, P.D. Netankar, R.A. Mane, Tetrahedron Lett. 52, 5817–5819 (2011)
A. Samadi, D. Silva, M. Chioua, L. Infantes, E. Soriano, J. Marco-Contelles, Mol. Divers. 19, 103–122 (2015)
Y. Peng, G. Song, Catal. Commun. 8, 111–114 (2007)
S. Asghari, R. Baharfar, M. Alimi, M. Ahmadipour, M. Mohseni, Monatsh. Chem. 145, 1337–1342 (2014)
T. Wejrzanowski, R. Pielaszek, A. Opalińska, H. Matysiak, W. Lojkowski, K.J. Kurzydlowski, Appl. Surf. Sci. 253, 204–208 (2006)
R. Pielaszek, J. Appl. Crystallogr. 1, 43–50 (2003)
Acknowledgments
This research was supported by the Research Council of the University of Mazandaran in Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Asghari, S., Mohammadnia, M. Synthesis and characterization of pyridine-4-carboxylic acid functionalized Fe3O4 nanoparticles as a magnetic catalyst for synthesis of pyrano[3,2-b]pyranone derivatives under solvent-free conditions. Res Chem Intermed 42, 1899–1911 (2016). https://doi.org/10.1007/s11164-015-2124-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2124-0