Abstract
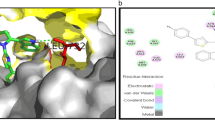
A series of new thiazolopyrimidine analogues were conveniently synthesized by one-pot multicomponent condensation reaction of ethyl acetoacetate, 2-aminothiazole and benzaldehyde substituted with different electron-donating and electron-withdrawing groups, in order to find some more potent antidiabetic and antibacterial drugs. The structures of the synthesized compounds were assigned based on elemental analyses and spectral data. An in vitro effect on total serum concentration of glucose, cholesterol and triglycerides was evaluated in adult male BALB/c mice, compared to two standard drugs “alloxan” and “glibenclamide,” and good results were observed with the presence of –Cl and –Br groups at the para position of the phenyl ring. The antibacterial activities were tested against five bacterial strains, Micrococcus luteus, Salmonella typhimurium, Bacillus subtilis, Bordetella bronchiseptica and Escherichia coli. Most of the compounds showed good to excellent bacterial zone inhibition compared to the reference drug “kanamycin.” An in silico molecular docking was also performed on synthesized compounds to support the experimental findings, which were in good agreement with computational results. The current study is expected to provide useful insights into the design of antidiabetic and antibacterial drugs, and understanding the mechanism by which such drugs interact with RNA and diabetes targets and exert their biochemical action.













Similar content being viewed by others
Abbreviations
- CVD:
-
Cardiovascular diseases
- CHD:
-
Comprising coronary heart
- T2DM:
-
Type 2 diabetes mellitus
- MI:
-
Myocardial infarction
References
M.K. Ali, K.M. Venkat, N. Tandon, Diabetes & coronary heart disease: current perspectives. Ind. J. Med. Res. 132, 584–597 (2010)
I.E.I.S. Hassan, H.Z. Viola, M.E.l.S Abdallah, A.E.l.B. Dina, Studies on the effects of bacterial diseases on skin and gill structure of Clarias gariepinus in Dakahlia Provinence Egypt. Ann. Biol. Res. 1(4), 106–118 (2010)
S.L. Croft, S. Sundar, A.H. Fairlamb, Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19, 111–126 (2006)
C.W. Muir, A.R. Kennedy, J.M. Redmond, A.J.B. Watson, Synthesis of functionalised 4H-quinolizin-4-ones via tandem Horner–Wadsworth–Emmons olefination/cyclisation. Org. Biomol. Chem. 11, 3337–3340 (2013)
N.H. Karam, J.H. Tomma, A.H. Al-Dujaili, Synthesis and characterization of new derivatives of thiazole with liquid crystalline properties. Chem. Mater. Res. 3(9), 162–171 (2013)
N.C. Desai, V.V. Joshi, K.M. Rajpara, H.V. Vaghani, H.M. Satodiya, Facile synthesis of novel fluorine containing pyrazole based thiazole derivatives and evaluation of antimicrobial activity. J. Fluor. Chem. 142, 67–78 (2012)
O.A.M. Fathalla, M.M. Anwar, M.E. Haiba, S.M. Nofal, Synthesis of novel tetrahydronaphthalen-2-yl heterocycles for analgesic, anti-inflammatory and antipyretic evaluation. Acta Pol. Pharm. 66, 259–270 (2009)
H.N. Karade, B.N. Acharya, M. Sathe, M.P. Kaushik, Design, synthesis and antimalarial evaluation of thiazole derived amino acids. Med. Chem. Res. 17, 19–29 (2008)
E. Brzezinska, G. Koska, A structure-activity relationship study of compounds with antihistamine activity. Biomed. Chromatogr. 20, 1004–1016 (2006)
J.S. Barradas, M.I. Errea, N.B. D’Accorso, C.S. Sepulveda, E.B. Damonte, Imidazo[2,1-b]thiazole carbohydrate derivatives: synthesis and antiviral activity against Junin virus, agent of Argentine hemorrhagic fever. Eur. J. Med. Chem. 46, 259–264 (2011)
R.A. Tapia, Y. Prieto, F. Pautet, N. Walchshofer, H. Fillion, B. Fenet, M.E. Sarciron, Synthesis and antiprotozoal evaluation of benzothiazolopyrroloquinoxalinones, analogues of kuanoniamine A. Bioorg. Med. Chem. 11, 3407–3412 (2003)
C.H. Oh, H.W. Cho, D. Baek, J.H. Cho, Synthesis and antibacterial activity of beta-methyl-2-(5-substituted thiazolo pyrrolidin-3-ylthio)carbapenem derivatives. Eur. J. Med. Chem. 37, 743–754 (2002)
S.K. Bharti, G. Nath, R. Tilak, S.K. Singh, Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur. J. Med. Chem. 45, 651–660 (2010)
L. Jaish, S.K. Srivastava, Synthesis and antimicrobial activity of some new N-methyl-piperazinylthiadiazoles and their azetidinones. J. Sci. Ind. Res. 60, 331–335 (2001)
U.P. Singh, H.R. Bhat, P. Gahtori, Antifungal activity, SAR and physicochemical correlation of some thiazole-1,3,5-triazine derivatives. J. Med. Mycol. 22(2), 134–141 (2012)
G.V.S. Kumar, Y. Rajendraprasad, B.P. Mallikarjuna, S.M. Chandrashekar, C. Kistayya, Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4 oxadiazoles as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 45, 2063–2074 (2010)
A. Marin, N. Valls, F.J. Berenguer, M.T. Alonso, A.M. Roman, M.M. Mercedes, J. Elguero, Synthesis and anthelmintic activity of carbamates derived from imidazo[2,1-b][1, 3, 4]thiadiazole and imidazo[2,1-b]thiazole. Farmaco (Societa Chimica Italiana) 47(1), 63–75 (1992)
A. Andreani, M. Rambaldi, A. Leoni, A. Locatelli, R. Bossa, M. Chiericozzi, I. Galatulas, G. Salvatore, Synthesis and cardiotonic activity of imidazo[2, l-b]thiazoles bearing a lactam ring. Eur. J. Med. Chem. 31(5), 383–387 (1996)
T.El-S. Ali, Synthesis and fungicidal activity of some new 4H-chromen-4-ones containing some 1,3-thiazole, 1,3-thiazine, 1,2,4-triazole and 1,2,4-triazine moieties. Phosphorus Sulfur Silicon Relat. Elem. 182(8), 1717–1726 (2007)
T. Honjo, G. Engelhardt, Effect of 2-amino-4,5,6,7-tetrahydro-5-propyl-thiazole(5,4-c)pyridine, an antihypertensive and sedative substance, on the noradrenaline storage in the heart of guinea pigs. Arzneimittelforschung 20(6), 845–850 (1970)
A. Geronikaki, Theophilidis, synthesis of 2-(aminoacetylamino)thiazole derivatives and comparison of their local anaesthetic activity by the method of action potential. Eur. J. Med. Chem. 27(7), 709–716 (1992)
A. Hu, Z. Qin, Y. J. Pingb, Synthesis, characterization and bactericidal activity of (E)-N-benzylidene-4-tert-butyl-5-(1,2,4-triazol-1-yl) thiazol-2-amines. Chin. J. Org. Chem. 30, 923–927 (2010)
T. Giridhar, R.B. Reddy, B. Prasanna, M.G.V.P. Chandra, Aminothiazoles: Part 1—syntheses and pharmacological evaluation of 4-[isobutylphenyl]-2-substitutedaminothiazoles. Indian J. Chem. 40B, 1279–1284 (2001)
M. Sobhi, G.K.D. Khalil, A convenient ultrasound-promoted synthesis of some new thiazole derivatives bearing a coumarin nucleus and their cytotoxic activity. Molecules 17, 9335–9347 (2012)
K. Anna, A. Dmytro, L. Roman, Thiopyrano[2,3-d]thiazole derivatives as potential anticancer agents. Sci. Pharm. 8(3), 509–529 (2012)
A.A. Kiryanov, P. Sampson, A.J. Seed, Synthesis of 2-alkoxy-substituted thiophenes, 1,3-thiazoles, and related S-heterocycles via Lawesson’s reagent-mediated cyclization under microwave irradiation: applications for liquid crystal synthesis. J. Org. Chem. 66, 7925–7929 (2001)
D. Vastag, S. Apostolov, M. Hadistevic, M. Sekulic, The possibility of copper corrosion protection in acidic media using a thiazole derivative. Mater. Technol. 47(3), 329–333 (2013)
C. Pifl, L. Pichler, W. Kobinger, O. Hornykiewicz, The dopamine autoreceptor agonist, B-HT 920, preferentially reduces brain dopamine release in vivo: biochemical indices of brain dopamine, noradrenaline and serotonin in ventriculocisternal perfusates in the cat. Eur. J. Pharm. 153(1), 33–44 (1988)
C.S. Schneider, J. Mierau, Dopamine autoreceptor agonists: resolution and pharmacological activity of 2,6-diaminotetrahydrobenzothiazole and an aminothiazole analog of apomorphine. J. Med. Chem. 30, 494–498 (1987)
J.M. Clark, S.J. Olsen, D.S. Weinberg, M. Dalvi, R.R. Whitney, D.P. Bonner, R.B. Sykes, In vivo evaluation of tigemonam, a novel oral monobactam. Antimicrob. Agents Chemother. 31(2), 226–229 (1987)
J.C. Eriks, H. Vandergoot, G.J. Sterk, H. Timmerman, Histamine H2-receptor agonists. Synthesis, in vitro pharmacology, and qualitative structure–activity relationships of substituted 4- and 5-(2-aminoethyl)thiazoles. J. Med. Chem. 35(17), 3239–3246 (1992)
C.E. Voogd, J.J. Van der Stel, H.W. Verharen, The capacity of some nitro and amino heterocyclic sulfur compounds to induce base-pair substitutions. Mutat. Res. 118(3), 153–165 (1983)
J.C. Greenaway, A.G. Fantel, M.R. Juchau, On the capacity of nitroheterocyclic compounds to elicit an unusual axial asymmetry in cultured rat embryos. Toxicol. Appl. Pharmacol. 82(2), 307–315 (1986)
S. Maddila, P. Lavanya, B. Sreekanth, V.Chunduri Jonnalagadda, Synthesis and antimicrobial studies of novel 2-benzylidene-phenylureido-thiazolopyrimidine derivatives. Chemija 23(2), 124–130 (2012)
Y. Guangfu, L. Huayin, Y. Xiufeng, Y. Huazheng, Design, syntheses and biological activity of novel CoMFA of sulfonylureas and triazolopyrimidine-2-sulfonamides ALS inhibitors. Sci. China (Ser. B) 42(6), 656–662 (1999)
A. Abdel-Aziem, M.S. El-Gendy, A.O. Abdelhamid, Synthesis and antimicrobial activities of pyrido[2,3-d]pyrimidine, pyridotriazolopyrimidine, triazolopyrimidine, and pyrido [2,3-d:6,5d′] dipyrimidine derivatives. Eur. J. Chem. 3(4), 455–460 (2012)
M.M. Youssef, M.A. Amin, Microwave assisted synthesis of some new thiazolopyrimidine, thiazolodipyrimidine and thiazolopyrimidothiazolopyrimidine derivatives with potential antioxidant and antimicrobial activity. Molecules 17, 9652–9667 (2012)
Y. Kotaiah, N.H. Krishna, K.N. Raju, C.V. Rao, S.B. Jonnalagadda, S. Maddila, Synthesis and biological evaluation of novel isopropyl 2-thiazolopyrimidine-6-carboxylate derivatives. J. Korean Chem. Soc. 56(1), 68–73 (2012)
X. Deng, S. Kokkonda, F.E.I. Mazouni, J. White, J.N. Burrows, W. Kaminsky, S.A. Charman, D. Matthews, P.K. Rathod, M.A. Phillips, Fluorine modulates species selectivity in the triazolopyrimidine class of plasmodium falciparum dihydroorotate dehydrogenase inhibitors. J. Med. Chem. 57, 5381–5394 (2014)
S. Maddila, G.L.V. Damu, E.O. Oseghe, O.A. Abafe, C.V. Rao, P. Lavanya, Synthesis and biological studies of novel biphenyl-3,5-dihydro-2H thiazolopyrimidines derivatives. J. Korean Chem. Soc. 56(3), 334–340 (2012)
I.Z. Qureshi, Q. Abbas, Modulation of testicular and whole blood trace element concentrations in conjunction with testosterone release following kisspeptin administration in male rabbits (Oryctolagus cuniculus). Biol. Trace Elem. Res. 154(2), 210–216 (2013)
M. Zaheer, A. Shah, Z. Akhter, R. Qureshi, B. Mirza, M. Tauseef, M. Bolte, Synthesis, characterization, electrochemistry and evaluation of biological activities of some ferrocenyl Schiff bases. Appl. Organomet. Chem. 25(1), 61–69 (2011)
S.G. Abdel Moty, M.A. Hussein, S.A.A.A. Aziz, M.A. Abou-Salim, Design and synthesis of some substituted thiazolo[3,2-a]pyrimidine derivatives of potential biological activities. Saudi Pharm. J. 22, 1–13 (2014). doi:10.1016/j.jsps.2013.12.016
E.X. Esposito, B. Eselli, K. Ken, D.M. Jeffry, Docking of sulfonamides to carbonic anhydrase II and IV. J. Mol. Graph. Model. 18(3), 283–289 (2000)
G.M. Morris, D.S. Goodsell, R. Huey, A.J. Olson, Distributed automated docking of flexible ligands to proteins: parallel applications of AutoDock 2.4. J. Comput. Aided Mol. Des. 10, 293–304 (1996)
M.K. Abdel-Hamid, A.A. Abdel-Hafez, N.A. EI-Koussi, N.M. Mahfouz, A. Innocenti, C.T. Supuran, Design, synthesis, and docking studies of new 1,3,4-thiadiazole-2-thione derivatives with carbonic anhydrase inhibitory activity. Bioorg. Med. Chem. 15(22), 6975–6984 (2007)
A. Saeed, P.A. Mahesar, S. Zaib, M.S. Khan, A. Matin, M. Shahid, J. Iqbal, Synthesis, cytotoxicity and molecular modeling studies of new phenylcinnamide derivatives as potent inhibitors of cholinesterases. Eur. J. Med. Chem. 78, 43–53 (2014)
S. Zaib, A. Saeed, K. Stolte, U. Flörke, M. Shahid, J. Iqbal, New aminobenzenesulfona mide-thiourea conjugates: synthesis and carbonic anhydrase inhibition and docking studies. Eur. J. Med. Chem. 78, 140–150 (2014)
P. Fouzia, Q. Rumana, S. Afzal, A. Safeer, L.A. Farzana, K. Saima, M. Sumera, Electrochemical, spectroscopic and molecular docking studies of anticancer organogermalactones. Int. Res. J. Pharm. 1(1), 1–8 (2011)
D.B. Kitchen, H. Decornez, J.R. Furr, J. Bajorath, Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov. 3, 935–949 (2004)
Acknowledgments
I.B. gratefully acknowledges a research scholarship from HEC Islamabad under the HEC Indigenous Ph.D. Scholarship 5000 Scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Batool, I., Saeed, A., Qureshi, I.Z. et al. Synthesis, molecular docking and biological evaluation of new thiazolopyrimidine carboxylates as potential antidiabetic and antibacterial agents. Res Chem Intermed 42, 1139–1163 (2016). https://doi.org/10.1007/s11164-015-2078-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2078-2