Abstract
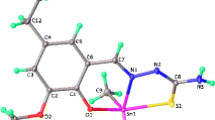
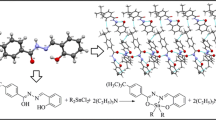
New organotin(IV) complexes of (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone thiosemicarbazone [L1H2], (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone phenylthiosemicarbazone [L2H2], and (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone semicarbazone [L3H2] with formula [R2SnL] (where R = Bu and Me) have been synthesized. The ligands and their organotin(IV) complexes were characterized by elemental analyses, molar conductivity, molecular weight determination, electronic, Fourier-transform infrared, and 1H, 13C, and 119Sn nuclear magnetic resonance spectral studies. The ligands act as tridentate and coordinate with organotin(IV) atom through the thiolate sulfur, azomethine nitrogen, and phenoxide oxygen atoms. The low molar conductance values in dimethylformamide indicate that the metal complexes are nonelectrolytes. Theoretical calculation is provided in support of the structures. The in vitro antimicrobial activities have been evaluated against Klebsiella sp., Bacillus cereus, Staphylococcus sp., Escherichia coli, Rhizopus, Aspergillus, Alternaria, and Penicillium. The screening results show that the organotin(IV) complexes have better antibacterial activities and have potential as drugs. Furthermore, it has been shown that dibutyltin(IV) derivative exhibits significantly better activity than the other organotin(IV) derivatives.







Similar content being viewed by others
References
C. Andreini, I. Bertini, G. Cavallaro, G.L. Holliday, J.M. Thornton, J. Biol. Inorg. Chem. 13, 1205–1218 (2008)
A.K. Cheetham, C.N.R. Rao, R.K. Feller, Chem. Commun. 46, 4780–4795 (2006)
C. Rodrigues, A.A. Batista, J. Ellena, E.E. Castellano, D. Benítez, H. Cerecetto, M. González, L.R. Teixeira, H. Beraldo, Eur. J. Med. Chem. 45, 2847–2853 (2010)
S.L.A. Kumar, M.S. Kumar, P.B. Sreeja, A. Sreekanth, Spectrochim. Acta Part A 113, 123–129 (2013)
O.K. Farha, J.T. Hupp, Acc. Chem. Res. 43, 1166–1175 (2010)
D.F. Weng, Z.M. Wang, S. Gao, Chem. Soc. Rev. 40, 3157–3181 (2011)
J.Y. Lee, O.K. Farha, J. Roberts, K.A. Scheidt, S.T. Nguyen, J.T. Hupp, Chem. Soc. Rev. 38, 1450–1459 (2009)
P. Paul, P. Sengupta, S. Bhattacharya, J. Organomet. Chem. 724, 281–288 (2013)
P. Paul, S. Datta, S. Halder, R. Acharyya, F. Basuli, R.J. Butcher, S.M. Peng, G.H. Lee, A. Castineiras, M.G.B. Drew, S. Bhattacharya, J. Mol. Catal. A: Chem. 344, 62–73 (2011)
M. Nath, M. Vats, P. Roy, Eur. J. Med. Chem. 59, 310–321 (2013)
F. Shaheen, A. Badshah, M. Gielen, G. Croce, U. Florke, U.D. de-Vos, S. Ali, J. Organomet. Chem. 695, 315–322 (2010)
M.X. Li, L.Z. Zhang, M. Yang, J.Y. Niu, J. Zhou, Bioorg. Med. Chem. Lett. 22, 2418–2423 (2012)
A. Karaküçük-İyidoğan, D. Taşdemir, E.E. Oruç-Emre, J. Balzarini, Eur. J. Med. Chem. 46, 5616–5624 (2011)
V.B. Arion, M.A. Jakupec, M. Galanski, P. Unfried, B.K. Keppler, J. Inorg. Biochem. 91, 298–305 (2002)
H.L. Singh, Spectrochim. Acta Part A 76, 253–258 (2010)
H.L. Singh, Res. Chem. Intermed. 37, 1087–1101 (2011)
K.S.O. Ferraz, L. Ferandes, D. Carrilho, M.C.X. Pinto, M.D.F. Leite, E.M. Souza-Fagundes, N.L. Speziali, I.C. Mendes, H. Beraldo, Bioorg. Med. Chem. 17, 7138–7144 (2009)
D.K. Demertzi, M.A. Demertzis, J.R. Miller, C. Papadopoulou, C. Dodorou, G. Filousis, J. Inorg. Biochem. 86, 555–563 (2001)
Y.F. Win, S.G. Teoh, T.S. Tengku-Muhammad, Y. Sivasothy, S.T. Ha, Am. J. Appl. Sci. 7, 301–308 (2010)
J.S. Casas, M.S. Garcra-Tasende, J. Sordo, Coord. Chem. Rev. 209, 197–261 (2000)
J.S. Casas, M.C. Rodrίguez-Argüelles, U. Russo, A. Sánchez, J. Sordo, A. Vázquez-López, S. Pinelli, P. Lunghi, A. Bonati, J.S. Casas, R. Albertini, J. Inorg. Biochem. 69, 283–292 (1998)
H.L. Singh, A.K. Varshney, Appl. Organomet. Chem. 15, 762–768 (2001)
K.S. Prasad, L.S. Kumar, M. Prasad, H.D. Revanasiddappa, Bioinorg. Chem. Appl. (2010). doi:10.1155/2010/854514
K.E. Apple, Drug Metabol. Rev. 36, 763–786 (2004)
M. Jain, S. Manju, R.V. Singh, Appl. Organomet. Chem. 18, 471–479 (2004)
G.L. Parrilha, J.G. da Silva, L.E. Gouveia, A.K. Gasparoto, R.P. Dias, W.R. Rocha, D.A. Santos, N.L. Speziali, H. Beraldo, Eur. J. Med. Chem. 46, 1473–1482 (2011)
M.X. Li, D. Zhang, L.Z. Zhang, J.Y. Niu, B.S. Ji, J. Organomet. Chem. 696, 852–858 (2011)
I.C. Mendes, F.B. Costa, G.M. de Lima, J.D. Ardisson, I. Garcia-Santos, A. Castiñeiras, H. Beraldo, Polyhedron 28, 1179–1185 (2009)
I.C. Mendes, J.P. Moreira, J.D. Ardisson, R.G. dos Santos, P.R.O. da Silva, I. Garcia, A. Castiñeiras, H. Beraldo, Eur. J. Med. Chem. 43, 1454–1461 (2008)
A. Perez-Rebolledo, J.D. Ayala, G.M. de Lima, N. Marchini, G. Bombieri, C.L. Zani, E.M. Souza-Fagundes, H. Beraldo, Eur. J. Med. Chem. 40, 467–472 (2005)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery, T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez, J.A. Pople, Gaussian 03, Revision C.01. (Gaussian, Inc., Wallingford CT, 2004)
H.L. Singh, J.B. Singh, K.P. Sharma, Res. Chem. Intermed. 38, 53–65 (2012)
M. Das, S.E. Livingstone, Inorg. Chim. Acta 19, 5–10 (1976)
B. Clarke, N. Clarke, D. Cunningham, T. Higgins, P. McArdle, M.N. Cholchuin, M. O’Gara, J. Organomet. Chem. 559, 56–64 (1998)
A.K. Saxena, J.K. Koacher, J.P. Tandon, Inorg. Nucl. Chem. Lett. 17, 229–233 (1981)
G.K. Sandhu, R. Gupta, S.S. Sandhu, R.V. Parish, K. Brown, J. Organomet. Chem. 279, 373–384 (1985)
M.T.H. Tarafder, A.R. Khan, Polyhedron 10, 819–822 (1991)
H.L. Singh, J.B. Singh, A. Mukharjee, Bioinorg. Chem. Appl. (2013). doi:10.1155/2013/425832
M. Nath, P.K. Saini, A. Kumar, J. Organomet. Chem. 695, 1353–1362 (2010)
H.L. Singh, J.B. Singh, Nat. Sci. 4, 170–178 (2012)
T.P. Lockhart, W.F. Manders, J. Am. Chem. Soc. 109, 7015–7020 (1987)
K.C. Molloy, P.C. Waterfield, J. Organomet. Chem. 424, 281–287 (1992)
A. Saxena, J.P. Tandon, A.J. Crowe, Polyhedron 4, 1085–1089 (1985)
A. Lycka, J. Holecek, S. Angelika, T. Ivan, J. Organomet. Chem. 409, 331–339 (1991)
T. Zoller, L. Iovkova-Berends, T. Berends, C. Dietz, G. Bradtmoller, K. Jurkschat, Inorg. Chem. 50, 8645–8653 (2011)
D.A. Atwood, J.A. Jegier, K.J. Martin, D. Rutherford, J. Organomet. Chem. 503, C4–C7 (1995)
G.F. de Sousa, V.M. Deflon, E. Niquet, A. Abras, J. Braz. Chem. Soc. 12, 493–498 (2001)
A.A. Al-Amiery, R.I. Al-Bayati, K.Y. Saour, M.F. Radi, Res. Chem. Intermed. 38, 745–759 (2012)
T.D. Thangadurai, K. Natarajan, Transit. Met. Chem. 26, 500–504 (2001)
I. Pal, F. Basuli, S. Bhattacharya, Proc. Indian Acad. Sci. Chem. Sci. 114, 255–268 (2002)
Y. Anjaneyula, R.P. Rao, Synth. React. Inorg. Met. Org. Chem. 16, 257–272 (1986)
Z.H. Chohan, A. Scozzafava, C.T. Supuran, J. Enzy. Inhib. Med. Chem. 18, 259–263 (2003)
K.S. Prasad, L.S. Kumar, S.C. Shekar, M. Prasad, H.D. Revanasiddappa, Chem. Sci. J. 12, 1–10 (2001)
N. Dharmaraj, P. Viswanathamurthi, K. Natarajan, Transit. Met. Chem. 26, 105–109 (2001)
A. Saxena, J.P. Tandon, Polyhedron 2, 443–446 (1983)
Acknowledgments
The authors are grateful to the Dean of Faculty of Engineering and Technology, Mody University of Science and Technology, Lakshmangarh, Sikar, for providing facilities necessary to carry out this research work. They are also grateful to Dr. T. Dewa, Department of Microbiology, University of Delhi, for providing antimicrobial screening facilities. The authors are also grateful to Dr. Sangeeta Jhajharia for linguistic corrections.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H.L., Singh, J.B. & Bhanuka, S. Synthesis, spectroscopic characterization, biological screening, and theoretical studies of organotin(IV) complexes of semicarbazone and thiosemicarbazones derived from (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone. Res Chem Intermed 42, 997–1015 (2016). https://doi.org/10.1007/s11164-015-2069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2069-3