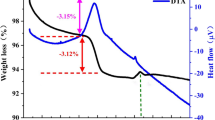
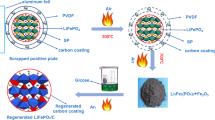
Abstract
According to the performance tests of LiFePO4 prepared by different iron sources, the product of Fe(NO3)3 had a pure phase and good electrochemical performances, while the raw material is cheaper, so Fe(NO3)3 was choosen as the iron source. The best determination of the experiment was that the lithium:iron ratio was 1, the carbon source content was 10 %, the sintering temperature was 700 °C for 6 h, and the sintering process was undertaken in a nitrogen atmosphere. The results showed that the initial discharge capacity of LiFePO4 synthesized in such conditions was 118 mAh/g in 2.5–4.2 V and at 0.1 C rate, and the capacity experienced almost no loss after 20 circulations.









Similar content being viewed by others
References
C. Masquelier, A.K. Padhi, K.S. Nanjundaswamy, J.B. Goodenough, New cathode materials for rechargeable lithium batteries: the 3-D framework structures Li3Fe2(XO4)3 (X P, As). J. Solid State Chem. 135, 228–234 (1998)
J.M. Tarascon, M. Armand, Nature 414, 359–367 (2001)
S.I. Nishimura, G. Kobayashi, K. Ohoyama, J. Kanno, M. Yashima, A. Yamada, Nat. Mater. (2008). doi:10.1038/nmat2251
A.K. Padhi, K.S. Nanjundaswamy, J.B. Goodenough, J. Electrochem. Soc. 144, 1188–1194 (1997)
J. Wang, X. Sun, Energy Environ. Sci. 5, 5163–5185 (2012)
K.F. Hsu, S.Y. Tsay, B.J. Hwang, Synthesis and characterization of nano-sized LiFePO4 cathode materials prepared by a citric acid-based sol–gel route. J. Mater. Chem. 14, 2690–2695 (2004)
Y. Wang, G. Cao, Developments in nanostructured cathode materials for high performance lithium-ion batteries. Adv. Mater. 20, 2257–2269 (2008)
I. Bilecka, A. Hintennach, I. Djerdj, P. Novak, M. Niederger, Efficient microwave assisted synthesis of LiFePO4 mesocrystals with high cycling stability. J. Mater. Chem. 19, 5125–5128 (2009)
M.H. Lee, J.Y. Kim, H.K. Song, A hollow sphere secondary structure of LiFePO4 nanoparticles. Chem. Commun. 46, 6795–6797 (2010)
J.Q. Zhao, J.P. He, J.H. Zhou, Y.X. Guo, T. Wang, S.C. Wu, X.H. Ding, R.M. Huang, H.R. Xue, Facile synthesis for LiFePO4 nanospheres in tridimensional porous carbon framework for lithium ion batteries. J. Chem. Phys. C 115, 2888–2894 (2011)
L.N. Wang, Z.G. Zhang, K.L. Zhang, A simple, cheap soft synthesis routine for LiFePO4 using iron (III) raw material. J. Power Source 167, 200–205 (2007)
K. Zaghib, A. Mauger, F. Gendron, C.M. Julien, Surface effects on the physical and electrochemical properties of thin LiFePO4 particles. Chem. Mater. 20, 462–469 (2008)
S.W. Oh, S.T. Maung, S.M. Oh, K.H. Oh, K. Amine, B. Scrosati, Y.K. Sun, Double carbon coating of LiFePO4 as high rate electrode for rechargeable lithium batteries. Adv. Mater. 22, 4842–4845 (2070)
M.S. Pan, Z.T. Zhou, Carbon rich surface of LiFePO4 grain enhancing its rate capability. Mater. Lett. 65, 1131–1133 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shao, ZC., Zhang, Y., Zhang, YX. et al. Effect of different sources of iron on the properties of LiFePO4/C for lithium-ion batteries. Res Chem Intermed 41, 3213–3222 (2015). https://doi.org/10.1007/s11164-013-1426-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1426-3