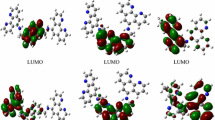
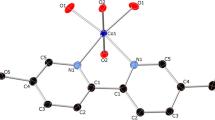
Abstract
The binuclear complex {[UO2(5,5′-dmbpy)(NO3)]2(μ-O2)}, where 5,5′-dmbpy is 5,5′-dimethyl-2,2′-bipyridine, was prepared by reaction of UO2(NO3)2.6H2O with 5,5′-dimethylbipyridine in 1:1 methanol–acetonitrile. The complex was characterized by FT-IR and UV–visible spectroscopy, cyclic voltammetry (CV), and single-crystal X-ray diffraction analysis. The crystal data for {[UO2(5,5′-dmbpy)(NO3)]2(μ-O2)} at 25 °C are: monoclinic, space group P2 1 /n with a = 9.8(7) Å, b = 16.0 (12) Å, c = 10.3 (8) Å, β = 111.023(9)° and Z = 2.




Similar content being viewed by others
References
R.K. Agarwal, I. Chakraborti, H. Agarwal, Synth. React. Inorg. Met. Org. Chem. 34, 1431 (2004)
R.K. Agarwal, I. Chakraborti, N.K. Sharma, J. Saudi Chem. Soc. 6, 41 (2002)
R.K. Agarwal, I. Chakraborti, H. Agarwal, Synth. React. Inorg. Met.-Org. Chem. 34, 1453 (2004)
A. Moghimi, M. Ranjbar, H. Aghabozorg, F. Jalili, M. Shamsipur, K.K. Chadha, Can. J. Chem. 80, 1687 (2002)
R.K. Agarwal, K. Arora, P. Garga, I. Chakraborti, J. Chem. 67, 1913 (1993)
R.K. Agarwal, K. Arora, P. Dutt, Synth. React. Inorg. Met. Org. Chem. 24, 301 (1994)
R.K. Agarwal, K. Arora, P. Dutt, Polyhedron 13, 957 (1994)
L. Singh, N. Tyagi, N.P. Dhaka, Asian J. Chem. 10, 915 (1998)
K. Arora, R.C. Goyal, S. Sharma, M.C. Pathak, Asian J. Chem. 11, 1005 (1999)
S. Lippard, J. Prog, Inorg. Chem. 8, 109 (1967)
E.L. Muetterties, J. Am. Chem. Soc. 88, 4856 (1966)
R.K. Agarwal, N. Tyagi, I. Chakraborti, Egypt. J. Anal. Chem. 7, 127 (1998)
S. Agnihotri, K. Arora, E.-J. Chem. 7(3), 1045 (2010)
A.R. de Aquinoa, P.C. Isolanib, J. Zukerman-Schpectorc, L.B. Zinnerb, G. Vicentinib, J. Alloy. Compd. 18, 323 (2001)
S.P. Pasilis, A. BlumenfeldInorg, Chemistry 50, 8302 (2011)
G.M. Sheldrick, SHELXTL, v. 5.10, Structure Determination Software Suite; (Bruker AXS, Madison, 1998)
K. Lonsdale, International Tables for X-ray Crystallography, vol. C (Kluwer Academic Publisher, Dordrecht, 1995)
V. Amani, N. Safari, H.R. Khavasi, Polyhedron 26, 4257 (2007)
V. Amani, N. Safari, H.R. Khavasi, M. Akkurt, Polyhedron 28, 3026 (2009)
K.W. Wellington, Synthetic and Analytical Studies of Biomimetric Metal Complexes, (Rhodes University, Grahamstown, 1999) [PhD Thesis]
K.W. Bagnal, O. Valasquez-Lopez, J. Chem. Soc. Dalton Trans. 27, 1409 (1975)
J.L. Sessler, D. Seidel, A.E. Vivian, V. Lynch, B.L. Scott, D.W. Keogh, Angew. Chem. Int. Ed. 40, 591 (2001)
A.R. de Aquino, G. Bombieri, P.C. Isolani, G. Vicentini, J. Zukerman-Schpector, Inorg. Chim. Acta 306, 102 (2000)
G. Szigethy, K.N. Raymond, J. Am. Chem. Soc. 133, 7942 (2011)
S.P. Pasilis, A. Blumenfeld, Inorg. Chem. 50, 8302 (2011)
D.L. Clark, S.D. Conradson, R.J. Donohoe, D.W. Keogh, D.E. Morris, P.D. Palmer, R.D. Rogers, C.D. Tait, Inorg. Chem. 38, 1456 (1999)
J.C. Arinze, N.E. Daniel, M.O.C. Ogwuegbu, J. Emerg. Trends. Eng. Appl. Sci. (JETEAS) 3(1), 61 (2012)
R. Krishna Reddy,. K. Suneetha, P. B. Karigar, C.S.C. Manjunath, K.N. Mahendra, J. Chil. Chem. Soc., 53, Nº 4 (2008)
Tokyo Institute of Technology Bulletin, Bulletin no. 16, May (2010)
M.L. Kantouri, Ch.D. Papadopoulos, M. Quiros, A.G. Hatzidimitriou, Polyhedron 26, 1292 (2007)
D.J. Szalda, Z.K. Creutz, D. Mahajan, N. Sutin, Inorg. Chem. 22, 2372 (1983)
B.P. Sallivan, D.J. Salmon, T.J. Meye, Inorg. Chem. 17, 3334 (1978)
T.R. Varga, A.C. Bényei, Z. Fazekas, H. Tomiyasu, Y. Ikeda, Inorg. Chim. Acta 342, 291 (2003)
V.S. Krivovichev, I.G. Tananaev, B.F. Myasoedov, Comptes Rendus Chimie 10, 897e904 (2007)
K. Chryssou, T. Stergiopoulos, P. Falaras, Polyhedron 21, 2773 (2002)
P. Giridhar et al., Radiochim. Acta 94, 415 (2006)
Acknowledgments
The authors sincerely thank the university of Sistan and Baluchestan for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akbarzadeh-T, N., Kondori, T. Synthesis, characterization, and crystal structure of a binuclear UO2(VI) complex with a bipyridine derivative ligand. Res Chem Intermed 41, 845–852 (2015). https://doi.org/10.1007/s11164-013-1236-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1236-7