Abstract
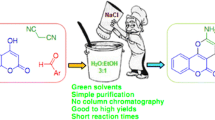
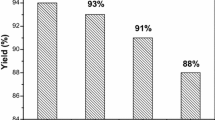
A novel and efficient one-pot synthesis of 10-aryl-7,8-dihydropyrano[3,2-b]chromene-4,9(6H,10H)-diones by three-component reaction of aromatic aldehydes, 5-hydroxy-2-methylpyran-4-one, and cyclic 1,3-dicarbonyl compounds in the presence of a catalytic amount of ceric ammonium nitrate under solvent-free conditions is described. The advantages of this method include operational simplicity, short reaction time, recyclable catalyst, and high yields.



Similar content being viewed by others
References
D.R. da Rocha, A.C.G. de Souza, J.A.L.C. Resende, W.C. Santos, E.A. dos Santos, C. Pessoa, M.O. de Moraes, L.V. Costa-Lotufo, R.C. Montenegro, V.F. Ferreira, Org. Biomol. Chem. 9, 4315 (2011)
S.B. Ferreira, F. de Carvalho da Silva, F.A.F.M. Bezerra, M.C.S. Lourenco, C.R. Kaiser, A.C. Pinto, V.F. Ferreira, Arch. Pharm. 343, 81 (2010)
M.Z. He, N. Yang, C.L. Sun, X.J. Yao, M. Yang, Med. Chem. Res. 20, 200 (2010)
A. Shahrisa, M. Zirak, A.R. Mehdipour, R. Miri, Chem. Heterocycl. Compd. 46, 1354 (2011)
R. Schiller, L. Tichotova, J. Pavlik, V. Buchta, B. Melichar, I. Votruba, J. Kunes, M. Spulak, M. Pour, Bioorg. Med. Chem. Lett. 20, 7358 (2010)
H. Hussain, S. Aziz, B. Schulz, K. Krohn, Nat. Prod. Commun. 6, 841 (2011)
S. Osman, B.J. Albert, Y.P. Wang, M.S. Li, N.L. Czaicki, K. Koide, Chem. Eur. J. 17, 895 (2011)
S.S. Bisht, N. Jaiswal, A. Sharma, S. Fatima, R. Sharma, N. Rahuja, A.K. Srivastava, V. Bajpai, B. Kumar, R.P. Tripathi, Carbohydr. Res. 346, 1191 (2011)
S.M. Wang, G.W.A. Milne, X.J. Yan, I. Posey, M.C. Nicklaus, L. Graham, W.G. Rice, J. Med. Chem. 39, 2047 (1996)
V.V. Mulwad, V.P. Kewat, Indian J. Heterocycl. Chem. 19, 47 (2009)
H. Miyazaki, K. Honda, M. Asami, S. Inoue, J. Org. Chem. 64, 9507 (1999)
J.S. Yadav, B.V.S. Reddy, L. Chandraiah, B. Jagannadh, S.K. Kumar, A.C. Kunwar, Tetrahedron Lett. 43, 4527 (2002)
B.V.S. Reddy, M.R. Reddy, G. Narasimhulu, J.S. Yadav, Tetrahedron Lett. 51, 5677 (2010)
W.L. Li, L.Q. Wu, L. Fu, J. Brazil, Chem. Soc. 22, 2202 (2011)
W.L. Li, J.Y. Liang, T.B. Wang, Y.Q. Wang, Collect. Czech. Chem. Commun. 76, 1791 (2011)
M.A.P. Martins, C.P. Frizzo, D.N. Moreira, L. Buriol, P. Machado, Chem. Rev. 109, 4140 (2009)
K. Tanaka, F. Toda, Chem. Rev. 100, 1025 (2000)
V. Sridharan, J.C. Menendez, Chem. Rev. 110, 3805 (2010)
Y.L. Li, B.X. Du, S.X. Wang, D.Q. Shi, S.J. Tu, J. Heterocycl. Chem. 43, 685 (2006)
Y.L. Li, X.P. Xu, D.Q. Shi, S.J. Ji, Chin. J. Chem. 27, 1510 (2009)
Y.L. Li, B.X. Du, X.P. Xu, D.Q. Shi, S.J. Ji, Chin. J. Chem. 27, 1563 (2009)
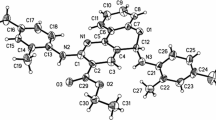
Crystallographic data for the structures of 4g reported in this paper have been deposited at the Cambridge Crystallographic Data Centre with No. CCDC-891132
Acknowledgments
We are grateful to the National Natural Science Foundation of China (no. 21104064, 21172188), the Natural Science Foundation of Xuzhou Normal University (10XLR03, 11XLR13) and the Priority Academic Program Development of Jiangsu Higher Education Institutions for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Meng, X., Cai, G. et al. CAN-catalyzed synthesis of 10-arylpyrano[3,2-b]chromene-4,9-diones under solvent-free conditions. Res Chem Intermed 40, 699–709 (2014). https://doi.org/10.1007/s11164-012-0995-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0995-x