Abstract
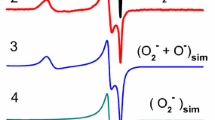
The concentration of O2 − radical anions generated on the surface of hydrated ZrO2 in an H2O2 solution was found to depend on H2O2 concentration. It was shown that this method can be used for detecting H2O2 in solutions at concentration as low as 0.01 wt%. The radical anions were found to react with organic molecules, even at room temperature. The decomposition kinetics of O2 − radical anions was double-exponential with two reaction rate constants. The existence of two distinct rate constants suggests that two types of O2 − radical anions with similar spectroscopic properties but different reactivity are present on the surface of hydrated ZrO2. It is highly likely that different arrangements of hydroxyl groups near the radical anions account for the presence of the two types of O2 − with different reactivity. The rate constants obtained in the presence of the organic compounds studied were found to conform with the expected order of reactivity: toluene > benzene ≫ hexane.





Similar content being viewed by others
References
M. Che, A.J. Tench, Adv. Catal. 31, 77 (1982)
M. Che, A.J. Tench, Adv. Catal. 32, 1 (1983)
A.M. Volodin, Catal. Today 58, 103 (2000)
R.M. Richards, A.M. Volodin, A.F. Bedilo, K.J. Klabunde, Phys. Chem. Chem. Phys. 5, 4299 (2003)
M. Sterrer, T. Berger, O. Diwald, E. Knozinger, A. Allouche, Top. Catal. 46, 111 (2007)
S.E. Malykhin, A.M. Volodin, A.F. Bedilo, G.M. Zhidomirov, J. Phys. Chem. C 113, 10350 (2009)
E. Giamello, M. Volante, B. Fubini, F. Geobaldo, C. Morterra, Mater. Chem. Phys. 29, 379 (1991)
M. Anpo, M. Che, B. Fubini, E. Garrone, E. Giamello, M.C. Paganini, Top. Catal. 8, 189 (1999)
A.F. Bedilo, M.A. Plotnikov, N.V. Mezentseva, A.M. Volodin, G.M. Zhidomirov, I.M. Rybkin, K.J. Klabunde, Phys. Chem. Chem. Phys. 7, 3059 (2005)
N.V. Mezentseva, A.F. Bedilo, A.M. Volodin, V.A. Sadykov, V.V. Lunin, Russ. J. Phys. Chem. 80, 1088 (2006)
E. Giamello, P. Rumori, F. Geobaldo, B. Fubini, M.C. Paganini, Appl. Magn. Reson. 10, 173 (1996)
S. Heinrich, M. Plettig, E. Klemm, Catal. Lett. 141, 251 (2011)
H. Kanai, M. Lintuluoto, Y. Matsumara, J.B. Moffat, J. Mol. Catal. A Chem. 252, 181 (2006)
A.F. Bedilo, A.M. Volodin, G.A. Zenkovets, G.V. Timoshok, React. Kinet. Catal. Lett. 55, 183 (1995)
A.V. Timoshok, A.F. Bedilo, A.M. Volodin, React. Kinet. Catal. Lett. 59, 165 (1996)
A.F. Bedilo, V.I. Kim, A.M. Volodin, J. Catal. 176, 294 (1998)
A.F. Bedilo, A.M. Volodin, Kinet. Catal. 50, 314 (2009)
A.F. Bedilo, A.S. Ivanova, N.A. Pakhomov, A.M. Volodin, J. Mol. Catal. A Chem. 158, 409 (2000)
A.F. Bedilo, K.J. Klabunde, Nanostruct. Mater. 8, 119 (1997)
A.F. Bedilo, K.J. Klabunde, J. Catal. 176, 448 (1998)
V.A. Bolshov, A.M. Volodin, G.M. Zhidomirov, A.A. Shubin, A.F. Bedilo, J. Phys. Chem. 98, 7551 (1994)
C. Morterra, E. Giamello, L. Orio, M. Volante, J. Phys. Chem. 94, 3111 (1990)
M. Occhiuzzi, D. Cordischi, R. Dragone, J. Phys. Chem. B 106, 12464 (2002)
D.A. Medvedev, A.A. Rybinskaya, R.M. Kenzhin, A.M. Volodin, A.F. Bedilo, Phys. Chem. Chem. Phys. 14, 2587 (2012)
Acknowledgments
Financial support by Russian Foundation for Basic Research (Grant 10-03-00691) is acknowledged with gratitude.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kenzhin, R.M., Volodin, A.M. & Bedilo, A.F. Reactivity of O2 − radical anions on hydrated ZrO2 surface. Res Chem Intermed 39, 1789–1797 (2013). https://doi.org/10.1007/s11164-012-0713-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0713-8