Abstract
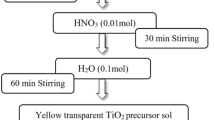
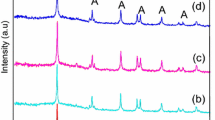
The nitrogen and iron(III) co-doped TiO2 (N–Fe–TiO2) samples were synthesized via modified sol–gel method by using alkyl amine as both nitrogen source and pore directing agent. Morphologies and properties of the co-doped TiO2 samples were investigated by X-ray diffraction, Fourier transform infrared spectroscopy, electron spin resonance spectroscopy, UV–Vis spectroscopy, scanning electron microscopy, and transmission electron microscopy. The results showed anatase phase mixed with rutile structure as well as hydroxyl and amine functional groups. The presence of Fe3+ in N–Fe–TiO2 sample was detected at g value of 2.00. In addition, the prepared samples were photocatalytically active for methyl orange degradation under UV light irradiation, but not under visible light.





Similar content being viewed by others
References
R.P.K. Wells, in Met. Oxide Catal., vol. 2, ed. by S.D. Jackson, J.S.J. Hargreaves (Wiley-VCH, London, 2009), p. 756
H. Choi, E. Stathatos, D.D. Dionysiou, Desalination 202, 199 (2007)
B. Naik, K.M. Parida, Ind. Eng. Chem. Res. 49, 8339 (2010)
S. Bouattour, A.M.B.D. Rego, L.F.V. Ferreira, Mater. Res. Bull. 45, 818 (2010)
C. Chem, W. Ma, J. Zhao, Curr. Org. Chem. 14, 630 (2010)
J.J. Zou, B. Zhu, L. Wang, X. Zhang, Z. Mi, J. Mol. Catal. A 286, 63 (2008)
Y. Cong, J. Zhang, F. Chen, M. Anpo, D. He, J. Phys. Chem. C 111, 10618 (2007)
H. Hao, J. Zhang, Microporous Mesoporous Mater. 121, 52 (2009)
J. Zhu, W. Zheng, B. He, J. Zhang, J. Mol. Catal. A 216, 35 (2004)
T. Tong, J. Zhang, B. Tian, F. Chen, D. He, J. Hazard. Mater. 155, 572 (2008)
F. Dong, H. Wang, Z. Wu, J. Qiu, J. Colloid Interface Sci. 343, 200 (2010)
Acknowledgments
This project is supported by the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative. Financial support from the Thailand Research Fund (TRF) and the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Education are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Songkhum, P., Tantirungrotechai, J. Synthesis, characterization, and catalytic activity of nitrogen and iron(III) co-doped TiO2 . Res Chem Intermed 39, 1555–1561 (2013). https://doi.org/10.1007/s11164-012-0620-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0620-z