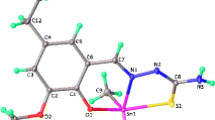
Abstract
Reaction of dibutyltin dichloride, dimethyltin dichloride, and tributyltin chloride with ligands derived from thiosemicarbazone and semicarbazone leads to the formation of a new series of organotin(IV) complexes of general formula R2SnCl2·L and R3SnCl·L (where L ligands derived from the condensation of thiosemicarbazide and semicarbazide with 4-hydroxy-3-methoxybenzaldehyde). The authenticity of these ligands and their metal complexes have been established on the basis of elemental analysis, conductance measurements, molecular weight determinations, infrared, 1H NMR, 13C NMR, 119Sn NMR, and UV spectral studies. These studies showed that the ligands coordinate to the metal atom in a bidentate. An octahedral structure is proposed for the organotin(IV) complexes. The ligands and its metal complexes are screened for their antimicrobial activities against some Gram-positive and Gram-negative bacteria, and fungus. The studies demonstrated that metalation can increase the antimicrobial activity rather than the free ligands.




Similar content being viewed by others
References
N.J. Walton, M.J. Mayer, A. Narbad, Phytochemistry 63, 505–515 (2003)
M.B. Hocking, J. Chem. Educ. 74, 1055–1059 (1997)
D.J. Fitzgerald, M. Stratford, M.J. Gasson, A. Narbod, J. Agric. Food Chem. 53, 1769–1775 (2005)
S.S. Konstantinovic, B.C. Radovanovic, S.P. Sovilj, S. Stanojevic, J. Serb. Chem. Soc. 73, 7–13 (2008)
A. Gonzalez, E. Gomez, A. Cortes-lozada, S. Hernandez, T. Ramirez-Apan, A. Nieto-Camacho, Chem. Pharm. Bull. 57, 5–15 (2009)
K.N. Kumar, R. Ramesh, Spectrochim. Acta. 60, 2913–2918 (2004)
M. Belicchi Ferrari, C. Pelizzi, G. Pelosi, M.C. Rodriguez, Polyhedron 21, 2593–2599 (2002)
H.L. Singh, Spectrochim. Acta. 76, 253–258 (2010)
S. Singh, F. Athar, M.R. Maurya, A. Azam, Eur. J. Med. Chem. 41, 592–598 (2006)
K.S. Abou Melha, J. Enzy, Inhib. Med. Chem. 23, 493–503 (2008)
M. Nath, R. Yadav, Bull. Chem. Soc. Jpn. 70, 1331–1337 (1997)
R. Willem, A. Bouhdid, B. Mahieu, L. Ghys, M. Biesemans, J. Organomet. Chem. 531, 151–158 (1997)
M. Gielen, M. Biesemans, D. de Vos, R. Willem, J. Inorg. Biochem. 79, 139–145 (2000)
H.L. Singh, A.K. Varshney, Appl. Organomet. Chem. 15, 762–768 (2001)
S.G. Teoh, S.H. Ang, S.B. Teo, H.K. Fun, K.L. Khew, J. Chem. Soc. Dalton Trans. 4, 465–468 (1997)
K.A. Crouse, K.-B. Chew, M.T.H. Harafder, A. Kaslsollah, A.M. Ali, B.M. Yamin, H.-K. Fun, Polyhedron 23, 161–168 (2004)
C.S. Parulekar, V.K. Jain, T. Kesavadas, E.R.T. Tiekink, J. Organomet. Chem. 387, 163–173 (1990)
S.R.A. Khan, S. Huang, S. Shamsuddin, S. Inutsuka, K.H. Whitmire, Bioorg. Med. Chem. 8, 515–521 (2000)
M.M. Amini, A. Azadmeher, V. Alijani, H.R. Khavazi, T. Hajiashrafi, Inorg. Chim. Acta 362, 355–360 (2009)
Y.F. Win, S.G. Teoh, E.K. Him, S.L. Ng, H.K. Fun, J. Chem. Crystallogr. 38, 345–350 (2008)
W.L.F. Armarego, C.L.L. Chai, Purification of Laboratory Chemicals, 5th edn. (Butterworth-Heinemann, London, 2003)
A. Rehman, M.I. Choudhary, W.J. Thomsen, Bioassay Techniques for Development (Harwood Academic Publishers, Amsterdam, 2001), p. 9
M.I. Fernandez, M. Fondo, A.M. Garcia-Deibe, B. Fernandez, M.J. Rodriguez, M.R. Bermego, Trans. Met. Chem. 27, 416–422 (2002)
I. Kizilcikli, Y.D. Kurt, B. Akkurt, A.Y. Genel, S. Birteksoz, G. Οtuk, B. Ulkuseven, Folia Microbiol. 52, 15–25 (2007)
H.L. Singh, M. Sharma, A.K. Varshney, Synth. React. Inorg. Met. Org. Chem. 29, 817–826 (1999)
H.L. Singh, Phosphorous Sulfur Silicon Relat. Elem. 184, 1768–1778 (2009)
V.B. Rana, P.C. Jain, M.P. Swami, A.K. Srivastava, J. Inorg. Nucl. Chem. 37, 1826–1828 (1975)
H.L. Singh, A.K. Varshney, Main Group Met. Chem. 22, 529–532 (1999)
H.C. Clark, V.K. Jain, R.C. Mehrotra, B.P. Singh, G. Srivastava, T. Birchall, J. Organomet. Chem. 279, 385–394 (1985)
J.S. Casas, M.S. Garcia-Tasende, J. Sordo, Coord. Chem. Rev. 209, 197–261 (2000)
G. Eng, X. Song, Q. Duong, D. Strickman, J. Glass, L. May, Appl. Organomet. Chem. 17, 218–225 (2003)
H.L. Singh, A.V. Varshney, Synthetic, structural, and biochemical studies of organotin(IV) with Schiff bases having nitrogen and sulphur donor ligands. Bioinorg. Chem. Appl. 2006, 7 (2006). doi:10.1155/BCA/2006/23245
A. Saxena, J.P. Tandon, Polyhedron 2, 443–446 (1983)
G. Bergamaschi, A. Bonardi, E. Leporati, P. Mazza, P. Pelagatti, C. Pelizzi, G. Pelizzi, M.C. Rodriguez-Argüelles, F. Zani, J. Inorg. Biochem. 68, 295–305 (1997)
H.L. Singh, B. Khungar, U.D. Tripaati, A.K. Varshney, Main Group Met. Chem. 24, 5–12 (2001)
Acknowledgments
The author is thankful to the dean of the Faculty of Engineering and Technology, Mody Institute of Technology and Science, Deemed University, Lakshmangarh, Sikar for providing the necessary facilities. The authors are also grateful to Dr. Ankit Gandhi for linguistic corrections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H.L., Singh, J.B. & Sharma, K.P. Synthetic, structural, and antimicrobial studies of organotin(IV) complexes of semicarbazone, thiosemicarbazone derived from 4-hydroxy-3-methoxybenzaldehyde. Res Chem Intermed 38, 53–65 (2012). https://doi.org/10.1007/s11164-011-0325-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-011-0325-8