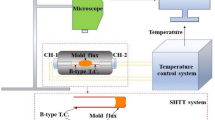
Mold powders (MP) are used as raw materials in steel smelting production. In this work TiO2, Na2CO3, MnO, and ZnO are used in a MP composition in order to reduce the CaF2 content or to replace it; CaF2 is used in order to prepare powder, similar to the commercial powder used in steel pouring. Agroove viscometer is used to study MP specimen viscosity, and x-ray diffraction XRD and scanning electron microscopy SEM are used for analyzing specimen behavior during crystallization. Results of studies show that there is cuspidine in XRD diffraction patterns of some specimens. It is established that glassy phase white crystals of cuspidine formed make it possible the regulate MP viscosity required for forming a lubricating layer between mold walls and a steel ingot shell. With use of MnO in MP in an amount of about 4 wt.% (apart from MnO within a specimen chemical composition) alongside F− in an amount of 2 wt.% low-fluorine MP specimens are prepared that may serve as a substitute for MP normally used in steel continuous casting.






Similar content being viewed by others
References
E. T. Turkdogan, Fundamentals of Steelmaking, The University Press, Cambridge (1996).
M. Mueller, W. Willenborg, K. Hilpert and L. Singheiser, “Structural dependence of alkali oxide activity in coal ash slag”, VII International Conference on molten slag, fluxes and salts, The South African Institute of Mining and Metallurgy (2004).
M. Nakamoto, J. Lee and T. Tanaka, “A model for estimation of molten silicate slag”, ISIJ Internat., 45(5), 651 – 656 (2005).
S. Sridhar, “Estimation models for molten slag and alloy viscosities”, JOM, 46 – 48 (2005).
R. F. Brooks, A. T. Dinsdale and P. N. Quested, “The measurement of viscosity of alloys — a review of methods, data and models”, Meas. Sci. Technol., 16, 354 – 362 (2005).
Q. Shu and J. Zhang, “Viscosity estimation for slags containing calcium fluoride”, J. Univ. Science and Technology Beijing, 12, 221 (2005.)
P. V. Riboud and M. Larrecq, “Fundamental study of the behavior of casting powders”, ISIJ Internat., 36, 522 – 525 (1996).
C. Orrling, A. W. Cramb, A. Tilliander, et al, “Observations of the melting and solidification behavior of mold slags”, Iron and Steelmaker, 27(1), 53 – 63 (2000).
S. Feldbauer, I. Jimbo, A. Sharan, K. Shimizu, and Y. Kashiwaya, “Physical properties of mold slags that are relevant to clean steel manufacture”, Proc. 78th Steelmaking Conference, Nashville, Iron & Steel Society (1995).
K. C. Mills, S. Sridhar, A. S. Normanton and S. T. Mallaband, “Mould flux behavior in continuous casting”, The Brimacombe Memorial Symposium (2000).
P. V. Riboud, Y. Roux, L. D. Lucas, et al, “Improvement of continuous casting powders”, Fachber. Hüttenprax. Metallweiterverarb., 19, 859 (1981).
G. Urbain, F. Cambier, M. Deletter and M. R. Anseau, “Viscosity of silicate melts”, Trans. J. British Ceramics Society, 80, 139 (1981).
E. T. Turkdogan, “Physicochemical properties of molten slags and glasses”, Metals Society, 11 (1983).
S. Seetgraman, D. Sichen, and F. Z. Ji, “Estimation of viscosities of ternary silicate melts using the excess Gibbs energy of mixing”, Met. Mater. Trans. B, 31B, 105 (2000).
L. Zhang and S. Jahanshahi, “Review and modeling of viscosity of silicate melts: Part I. Viscosity of binary and ternary silicates containing CaO, MgO and MnO”, Met. Mater. Trans. B, 31B, issue.1, 177 (1998).
M. K. Koul, S. Sankaranarayanan, D. Apelian,W. L. McCauley, Mould Powder Technology, Press of Northeast University of Technology, (1988).
A. Morita, T. Omoto and Y. Iwamoto, United States Patent, No. US00641402B1, Molding powder for continuous casting of steel and a method for continuous casting of steel (2002).
W. En-fa, Y. Yin-dong, F. Chang-lin, et al., “Effect of carbon properties on melting Behavior of mold fluxes for continuous casting of steels”, J. Iron and Steel Res. International, 13(2), 22 – 26 (2006).
G. Wen, S. Sridhar, P. Tang, et al., “Development of fluoridefree mold powders for peritectic steel slab casting”, ISIJ Internat., 47, 1117 – 1125 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 5, pp. 35 – 41, May, 2013.
Rights and permissions
About this article
Cite this article
Arefpour, A.R., Monshi, A., Saidi, A. et al. Effect of CaF2 and MnO on Mold Powder Viscosity and Solidification During High-Speed Continuous Casting. Refract Ind Ceram 54, 203–209 (2013). https://doi.org/10.1007/s11148-013-9575-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-013-9575-x