Abstract
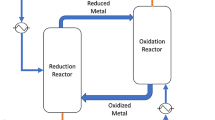
10%NiO–La0.3Sr0.7Co0.7Fe0.3O3−δ (10%NiO-LSCF3773) was synthesized using the EDTAcitrate complexing method. Non-catalytic and catalytic nitrous oxide decomposition and methane partial oxidation using 10%NiO–LSCF3773 was experimentally studied, assuming that the reactions occurred separately in a membrane reactor at feed side and permeate side. The experimental results are in good agreement with the chemical equilibrium composition calculated using Aspen Plus, and the changes of standard Gibbs free energy of each relevant elementary reactions. The mechanism of the reactions was proposed to follow Eley–Rideal surface reaction. The optimal temperature was 800 °C, under atmospheric pressure, where (1) NO2 formation was not detected (2) no production of C2 + and C3 + (3) complete conversion of N2O, CH4 and O2 were achieved (4) high purity syngas was obtained with no significant amount of undesired products and (5) readily utilizable syngas at the ratio of two was achieved.








Similar content being viewed by others
References
Li C, Shen Y, Zhu S, Shen S (2014) Supported Ni–La–Ox for catalytic decomposition of N2O I: component optimization and synergy. RSC Adv 4(55):29107
Xue L, Zhang C, He H, Teraoka Y (2007) Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl Catal B 75(3–4):167–174
Liu Z, Zhou Z, He F, Chen B, Zhao Y, Xu Q (2017) Catalytic decomposition of N2O over NiO–CeO2 mixed oxide catalyst. Catal Today 293–294:56–60
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilmen DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
van den Brink RW, Booneveld S, Verhaak MJFM, De Bruijn FA (2002) Selective catalytic reduction of N2O and NOx in a single reactor in the nitric acid industry. Catal Today 75:227–232
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326(5949):123–125
Li L, Xu J, Hu J, Han J (2014) Reducing nitrous oxide emissions to mitigate climate change and protect the ozone layer. Environ Sci Technol 48(9):5290–5297
Pedros PB, Askari O, Metghalchi H (2016) Reduction of nitrous oxide emissions from biological nutrient removal processes by thermal decomposition. Water Res 106:304–311
Abu-Zied BM, Schwieger W, Unger A (2008) Nitrous oxide decomposition over transition metal exchanged ZSM-5 zeolites prepared by the solid-state ion-exchange method. Appl Catal B 84(1–2):277–288
Rivallan M, Ricchiardi G, Bordiga S, Zecchina A (2009) Adsorption and reactivity of nitrogen oxides (NO2, NO, N2O) on Fe-zeolites. J Catal 264(2):104–116
Khan NA, Kennedy EM, Dlugogorski BZ, Adesina AA, Stockenhuber M (2014) Partial oxidation of methane with nitrous oxide forms synthesis gas over cobalt exchanged ZSM-5. Catal Commun 53:42–46
Ruiz-Trejo E, Boldrin P, Medley-Hallam JL, Darr J, Atkinson A, Brandon NP (2015) Partial oxidation of methane using silver/gadolinia-doped ceria composite membranes. Chem Eng Sci 127:269–275
Indarto A (2016) Partial oxidation of methane to methanol with nitrogen dioxide in dielectric barrier discharge plasma: experimental and molecular modeling. Plasma Sources Sci Technol 25(2):025002
Lee S-J, Ryu I-S, Kim B-M, Moon S-H (2011) A review of the current application of N2O emission reduction in CDM projects. Int J Greenhouse Gas Control 5(1):167–176
Zeng HC, Pang XY (1997) Catalytic decomposition of nitrous oxide on alumina-supported ruthenium catalysts Ru/Al2O3. Appl Catal B 13:113–122
Slagterna Å, Swaan HM, Olsbyea U, Dahla IM, Mirodatosb C (1998) Catalytic partial oxidation of methane over Ni-, Co- and Fe-Based catalysts. Catal Today 46:107–115
Yuzaki K, Yarimizu T, Aoyagi K, Ito S, Kunimori K (1998) Catalytic decomposition of N2O over supported Rh catalysts: effects of supports and Rh dispersion. Catal Today 45:129–134
Marnasidou KG, Voutetakis SS, Tjatjopoulos GJ, Vasalos IA (1999) Catalytic partial oxidation of methane to synthesis gas in a pilot-plant-scale spouted-bed reactor. Chem Eng Sci 54:3691–3699
Ohno Y, Kimura K, Bi M, Matsushima T (1999) The translational energy of desorbing products in NO and N2O decomposition on Pd (110). J Chem Phys 110(17):8221–8224
Huang C, Zhu Y, Wang X, Liu X, Wang J, Zhang T (2017) Sn promoted BaFeO3-δ catalysts for N2O decomposition: optimization of Fe active centers. J Catal 347:9–20
Li K, Wang XF, Zeng HC (1997) Kinetics of N2O Decomposition on a RuO2/Al2O3 Catalyst. Chem Eng Res Des 75(8):807–812
Zhu J, Vanommen J, Lefferts L (2006) Effect of surface OH groups on catalytic performance of yittrium-stabilized ZrO2 in partial oxidation of CH4 to syngas. Catal Today 117(1–3):163–167
Fleys M, Shan W, Simon Y, Marquaire P (2007) Detailed kinetic study of the partial oxidation of methane over La2O3 catalyst. Part 1: experimental results. Ind Eng Chem Res 46:1063–1068
Fleys M, Simon Y, Marquaire P (2007) Detailed kinetic study of the partial oxidation of methane over La2O3 catalyst. Part 2: mechanism. Ind Eng Chem Res 46:1069–1078
Dacquin JP, Lancelot C, Dujardin C, Da Costa P, Djega-Mariadassou G, Beaunier P, Kaliaguine S, Vaudreuil S, Royer S, Granger P (2009) Influence of preparation methods of LaCoO3 on the catalytic performances in the decomposition of N2O. Appl Catal B 91(3–4):596–604
Wu Y, Cordier C, Berrier E, Nuns N, Dujardin C, Granger P (2013) Surface reconstructions of LaCo1-xFexO3 at high temperature during N2O decomposition in realistic exhaust gas composition: impact on the catalytic properties. Appl Catal B 140–141:151–163
Ivanov DV, Pinaeva LG, Sadovskaya EM, Isupova LA (2016) Isotopic transient kinetic study of N2O decomposition on LaMnO3+δ. J Mol Catal A 412:34–38
Yang Z, Zhang Y, Ding W (2014) Investigation on the reforming reactions of coke-oven-gas to H2 and CO in oxygen-permeable membrane reactor. J Membr Sci 470:197–204
Cheng H, Lu X, Hu D, Zhang Y, Ding W, Zhao H (2011) Hydrogen production by catalytic partial oxidation of coke oven gas in BaCo0.7Fe0.2Nb0.1O3-δ membranes with surface modification. Int J Hydrogen Energy 36(1):528–538
Zhu X, Li Q, He Y, Cong Y, Yang W (2010) Oxygen permeation and partial oxidation of methane in dual-phase membrane reactors. J Membr Sci 360(1–2):454–460
Song S, Zhang P, Han M, Singhal SC (2012) Oxygen permeation and partial oxidation of methane reaction in Ba0.9Co0.7Fe0.2Nb0.1O3-δ oxygen permeation membrane. J Membr Sci 415–416:654–662
Jin W, Li S, Huang P, Xu N, Shi J, Lin YS (2000) Tubular lanthanum cobaltite perovskite-type membrane reactors for partial oxidation of methane to syngas. J Membr Sci 166:13–22
Li S, Jin W, Huang P, Xu N, Shi J, Lin YS (2000) Tubular lanthanum cobaltite perovskite type membrane for oxygen permeation. J Membr Sci 166:51–61
Figen HE, Baykara SZ (2018) Effect of ruthenium addition on molybdenum catalysts for syngas production via catalytic partial oxidation of methane in a monolithic reactor. Int J Hydrogen Energy 43(2):1129–1138
Swamy CS, Christopher J (1992) Decomposition of N2O on perovskite-related oxides. Catal Rev 34(4):409–425
Babiniec SM, Coker EN, Miller JE, Ambrosini A (2015) Investigation of LaxSr1-xCoyM1-yO3-δ (M = Mn, Fe) perovskite materials as thermochemical energy storage media. Sol Energy 118:451–459
Koh A, Chen L, Keeleong W, Johnson B, Khimyak T, Lin J (2007) Hydrogen or synthesis gas production via the partial oxidation of methane over supported nickel-cobalt catalysts. Int J Hydrogen Energy 32(6):725–730
Kangsadan T, Srisurat T, Kim P, Laosiripojana N, Jindasuwan S, Hartley UW (2015) Hydrogen production from palmitic acid through autothermal reforming: thermodynamic analysis. Eng J 19(4):153–165
Zhang J, Haribal V, Li F (2017) Perovskite nanocomposites as effective CO2-splitting agents in a cyclic redox scheme. Sci Adv 3(8):e1701184
Kapteijn F, RodriguezMirasol J, Moulijn JA (1996) Heterogeneous catalytic decomposition of nitrous oxide. Appl Catal B 9:25–64
Smith JM, Van Ness HC, Abbott MM (2005) Introduction to chemical engineering thermodynamics, 7th edn. McGraw Hill, New York
Felder RM, Rousseau RW (2005) Elementary principles of chemical processes, 3rd edn. Wiley, New York
Yaws CL (1996) Handbook of thermodynamics diagram. Gulf Publishing Company, Houston
Standard thermodynamic properties of chemical substances.
Speight JG (2017) Rules of thumb for petroleum engineers, 1st edn. Wiley, Hoboken
Pavlova S, Kapokova L, Bunina R, Alikina G, Sazonova N, Krieger T, Ishchenko A, Rogov V, Gulyaev R, Sadykovab V, Mirodatosc C (2012) Syngas production by CO2 reforming of methane using LnFeNi(Ru)O3 perovskites as precursors of robust catalysts. Catal Sci Technol 2:2099–2108
Xu X, Li L, Yu F, Peng H, Fang X, Wang X (2017) Mesoporous high surface area NiO synthesized with soft templates: remarkable for catalytic CH4 deep oxidation. Mol Catal 441:81–91
Bai G, Dai H, Deng J, Liu Y, Qiu W, Zhao Z, Li X, Yang H (2013) The microemulsion preparation and high catalytic performance of mesoporous NiO nanorods and nanocubes for toluene combustion. Chem Eng J 219:200–208
Araujo GCd, Lima SMd, Assaf JM, Peña MA, Fierro JLG, do Carmo Rangel M (2008) Catalytic evaluation of perovskite-type oxide LaNi1-xRuxO3 in methane dry reforming. Catal Today 133–135:129–135
Shan W (2003) Reduction property and catalytic activity of Ce1-XNiXO2 mixed oxide catalysts for CH4 oxidation. Appl Catal A General 246(1):1–9
Cihlar J, Vrba R, Castkova K, Cihlar J (2017) Effect of transition metal on stability and activity of La-Ca-M-(Al)-O (M = Co, Cr, Fe and Mn) perovskite oxides during partial oxidation of methane. Int J Hydrogen Energy 42(31):19920–19934
Zhao K, Zheng A, Li H, He F, Huang Z, Wei G, Shen Y, Zhao Z (2017) Exploration of the mechanism of chemical looping steam methane reforming using double perovskite-type oxides La1.6Sr0.4FeCoO6. Appl Catal B 219:672–682
Li R, Yu C, Shen S (2002) Partial oxidation of methane to syngas using lattice oxygen of La1-xSrxFeO3 perovskite oxide catalysts instead of molecular oxygen. J Nat Gas Chem 117:137–144
Singha RK, Shukla A, Yadav A, Sivakumar Konathala LN, Bal R (2017) Effect of metal-support interaction on activity and stability of Ni-CeO2 catalyst for partial oxidation of methane. Appl Catal B 202:473–488
Zheng Y, Li K, Wang H, Tian D, Wang Y, Zhu X, Wei Y, Zheng M, Luo Y (2017) Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane. Appl Catal B 202:51–63
Pal P, Singha RK, Saha A, Bal R, Panda AB (2015) Defect-induced efficient partial oxidation of methane over nonstoichiometric Ni/CeO2 nanocrystals. J Phys Chem C 119(24):13610–13618
Acknowledgements
Grants from Thailand Research Fund (MSD5910030) and National Research Council of Thailand (KMUTNB-GOV-58-54) and (KMUTNB-GOV-59-43) are acknowledged for the support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khajonvittayakul, C., Tongnan, V., Kangsadan, T. et al. Thermodynamic and mechanism study of syngas production via integration of nitrous oxide decomposition and methane partial oxidation in the presence of 10%NiO–La0.3Sr0.7Co0.7Fe0.3O3−δ. Reac Kinet Mech Cat 127, 839–855 (2019). https://doi.org/10.1007/s11144-019-01600-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01600-1