Abstract
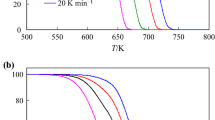
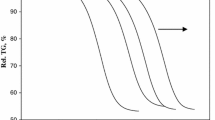
The possibility of fitting the non-isothermal kinetics of C60(OH)27 dehydroxylation by the Frazer–Suzuki equation has been investigated. Thermogravimetric curves of fullerol dehydroxylation have been recorded at different heating rates ranging from 5 to 25 K min−1. The curves of fullerol dehydroxylation reaction rate were completely deconvoluted with the two Frazer–Suzuki functions at all heating rates and hence, it was concluded that mechanism of C60(OH)27 dehydroxylation consisted from two dehydroxylation reactions. Reaction components can be connected with the existence of two clusters on fullerene surface that are formed from OH groups. Using Vyazovkin’s isoconversional method, it was found that, for both components, apparent activation energy changes with dehydroxylation degree, meaning that deconvolution using the Frazer–Suzuki function do not decomposes complex reaction of fullerol dehydroxylation on elementary steps. The dependence of activation energies on their dehydroxylation degree for each of the reaction components is the consequence of the existence of activation energy distribution in the form of a narrow peak, which can be further related with unique reaction model function for each dehydroxylation component.











Similar content being viewed by others
References
Kokubo K (2012) Water-soluble single-nano carbon particles: fullerenol and its derivatives. In: Hashim AA (ed) The delivery of nanoparticles. InTech, Rijeka
Chaudhuri P, Paraskar A, Soni S et al (2009) Fullerenol-cytotoxic conjugates for cancer chemotherapy. ACS Nano 3:2505–2514. https://doi.org/10.1021/nn900318y
Wang Z, Lu Z, Zhao Y, Gao X (2015) Oxidation-induced water-solubilization and chemical functionalization of fullerenes C60, Gd@C60 and Gd@C82: atomistic insights into the formation mechanisms and structures of fullerenols synthesized by different methods. Nanoscale 7:2914–2925
Aoshima H, Kokubo K, Shirakawa S, Ito M, Yamana S, Oshima T (2009) Antimicrobial activity of fullerenes and their hydroxylated derivatives. Biocontrol Sci 14:69–72
Krishna V, Singh A, Sharma P, Iwakuma N, Wang Q, Zhang Q, Knapik J, Jiang H, Grobmyer SR, Koopman B, Moudgil B (2010) Polyhydroxy fullerenes for non-invasive cancer imaging and therapy. Small 6:2236–2241
Sun Y-B, Cao C-Y, Yang S-L, Huang P-P, Wang C-R, Song W-G (2014) C60 fullerenol as an active and stable catalyst for the synthesis of cyclic carbonates from CO2 and epoxides. Chem Commun 50:10307–10310
Mohan H, Palit DK, Mittal JP, Chiang LY, Asmus K-D, Guldi DM (1998) Excited states and electron transfer reactions of C60(OH)18 in aqueous solution. J Chem Soc Faraday Trans 94:359–363
Torres VM, Srdjenovic B, Jacevic V, Simic VD, Djordjevic A, Simplício AL (2010) Fullerenol C60(OH)24 prevents doxorubicin-induced acute cardiotoxicity in rats. Pharmacol Rep PR 62:707–718
Marcorin GL, Da Ros T, Castellano S, Stefancich G, Bonin I, Miertus S, Prato M (2000) Design and synthesis of novel [60]fullerene derivatives as potential hiv aspartic protease inhibitors. Org Lett 2:3955–3958
Mashino T, Shimotohno K, Ikegami N, Wang Q, Zhang Q, Knapik J, Jiang H, Grobmyer SR, Koopman B, Moudgil B (2005) Human immunodeficiency virus-reverse transcriptase inhibition and hepatitis C virus RNA-dependent RNA polymerase inhibition activities of fullerene derivatives. Bioorg Med Chem Lett 15:1107–1109
Lu LH, Lee YT, Chen HW, Chiang LY, Huang HC (1998) The possible mechanisms of the antiproliferative effect of fullerenol, polyhydroxylated C60, on vascular smooth muscle cells. Br J Pharmacol 123:1097–1102
Chiang LY, Upasani RB, Swirczewski JW, Soled S (1993) Evidence of hemiketals incorporated in the structure of fullerols derived from aqueous acid chemistry. J Am Chem Soc 115:5453–5457
Goswami TH, Singh R, Alam S, Mathur GN (2004) Thermal analysis: a unique method to estimate the number of substituents in fullerene derivatives. Thermochim Acta 419:97–104
Gao XJ, Shen X, Chen B-Z, Gao X (2016) Improved description for the structures of fullerenols C60(OH)n (n = 12–48) and C2v(9)-C82(OH)x (x = 14–58). J Phys Chem C 120:11709–11715
Perejón A, Sánchez-Jiménez PE, Criado JM, Pérez-Maqueda LA (2011) Kinetic analysis of complex solid-state reactions. a new deconvolution procedure. J Phys Chem B 115:1780–1791
Adnađević B, Gigov M, Sindjic M, Jovanović J (2008) Comparative study on isothermal kinetics of fullerol formation under conventional and microwave heating. Chem Eng J 140:570–577
Georgieva AT, Pappu V, Krishna V et al (2013) Polyhydroxy fullerenes. J Nanoparticle Res 15:1690
Xing G, Zhang J, Zhao Y et al (2004) Influences of structural properties on stability of fullerenols. J Phys Chem B 108:11473–11479
Vyazovkin S (2001) Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem 22:178–183
Flynn JH (1997) The ‘temperature integral’—its use and abuse. Thermochim Acta 300:83–92
Vand V (1943) A theory of the irreversible electrical resistance changes of metallic films evaporated in vacuum. Proc Phys Soc A55:222–246
Miura K (1995) A new and simple method to estimate f(E) and k0(E) in the distributed activation energy model from three sets of experimental data. Energy Fuels 9(2):302–307
Miura K, Maki T (1998) A simple method for estimating f(E) and k0(E) in the distributed activation energy model. Energy Fuels 12(5):864–869
Khawam A, Flanagan DR (2006) Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B 110:17315–17328
Vyazovkin S, Wight CA (1997) Kinetics in solids. Annu Rev Phys Chem 48:125–149
Liu NA, Chen HX (2007) Two-step kinetics models for thermal decomposition of forest combustibles: three kinetics schemes. Fire Safety Sci AOFST 7:56–68
Cheng Z, Wu W, Ji P et al (2015) Applicability of Fraser-Suzuki function in kinetic analysis of DAEM processes and lignocellulosic biomass pyrolysis processes. J Therm Anal Calorim 119:1429–1438
Stankovic B, Jovanovic J, Ostojic S, Adnadjevic B (2017) Kinetic analysis of non-isothermal dehydration of poly(acrylic acid)-g-gelatin hydrogel using distributed activation energy model. J Therm Anal Calorim 129:541–551
Acknowledgements
This investigation was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia trough Project 172015OI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stankovic, B., Jovanovic, J. & Adnadjevic, B. Application of the Suzuki–Fraser function in modelling the non-isothermal dehydroxylation kinetics of fullerol. Reac Kinet Mech Cat 123, 421–438 (2018). https://doi.org/10.1007/s11144-018-1380-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-018-1380-6