Abstract
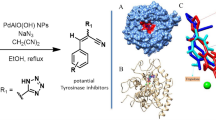
When the action of tyrosinase on l-dopa in the presence of 3-aminoacetophenone is studied, inhibition or activation is observed depending on the concentration of substrate. These opposing effects are due to non-enzymatic reactions: when tyrosinase acts on l-dopa, a highly reactive molecule, o-dopaquinone, originates, which evolves to dopachrome; in the presence of 3-/4-aminoacetophenones, the amino group of these compounds nucleophilically attacks the o-quinones, generating a colorless adduct, which, in turn, is oxidized by another molecule of o-dopaquinone, giving a colored compound with an absorbance higher than that of the dopachrome in an apparent activating effect. Therefore, an apparent activation or inhibition is seen depending on the concentration of l-dopa used in the experiments. Moreover, docking studies showing the binding mode of these molecules to the met (E m) and oxy (E ox) forms of tyrosinase reveal that both compounds arranges itself in the active centre in a similar way to monophenolic substrates of the enzyme, confirming the inhibition of the enzyme. We proposed a kinetic test to elucidate whether a compound is a true activator or an inhibitor of tyrosinase.






Similar content being viewed by others
References
Wang N, Hebert DN (2006) Tyrosinase maturation through the mammalian secretory pathway: bringing color to life. Pigm Cell Res 19:3–18
Sánchez-Ferrer A, Rodríguez-López JN, García-Cánovas F, García-Carmona F (1995) Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta 1247:1–11
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96:2563–2605
Yim TK, Wu WK, Mak DH, Ko KM (1998) Myocardial protective effect of an anthraquinone-containing extract of Polygonum multiflorum ex vivo. Planta Med 64:607–611
Imokawa G, Yada Y, Miyagishi M (1992) Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem 267:24675–24680
Pang Y, Geng J, Qin Y, Wang H, Fan R, Zhang Y, Li H, Jiang S, Dong C (2016) Endothelin-1 increases melanin synthesis in an established sheep skin melanocyte culture. In Vitro Cell Dev Biol-Anim 52:749
Guan S, Su W, Wang N, Li P, Wang Y (2008) A potent tyrosinase activator from Radix Polygoni multiflori and its melanogenesis stimulatory effect in B16 melanoma cells. Phytother Res 22:660–663
Shabani F, Sariri R (2010) Increase of melanogenesis in the presence of fatty acids. Pharmacologyonline 1:314–323
Takekoshi S, Matsuzaki K, Kitatani K (2013) Quercetin stimulates melanogenesis in hair follicle melanocyte of the mouse. Tokai J Exp Clin Med 38:129–134
Takekoshi S, Nagata H, Kitatani K (2014) Flavonoids enhance melanogenesis in human melanoma cells. Tokai J Exp Clin Med 39:116–121
Briganti S, Camera E, Picardo M (2003) Chemical and instrumental approaches to treat hyperpigmentation. Pigm Cell Res 16:101–110
Parvez S, Kang M, Chung HS, Bae H (2007) Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytother Res 21:805–816
Lee SY, Baek N, Nam TG (2016) Natural, semisynthetic and synthetic tyrosinase inhibitors. J Enzym Inhib Med Chem 31:1–13
Chang TS (2009) An updated review of tyrosinase inhibitors. Int J Mol Sci 10:2440–2475
Khan MT (2012) Novel tyrosinase inhibitors from natural resources—their computational studies. Curr Med Chem 19:2262–2272
Solano F (2016) Photoprotection versus photodamage: updating an old but still unsolved controversy about melanin. Polym Int. doi:10.1002/pi.5117
You A, Zhou J, Song S, Zhu G, Song H, Yi W (2015) Structure-based modification of 3-/4-aminoacetophenones giving a profound change of activity on tyrosinase: from potent activators to highly efficient inhibitors. Eur J Med Chem 93:255–262
Garcia-Molina F, Munoz JL, Varon R, Rodriguez-Lopez JN, Garcia-Canovas F, Tudela J (2007) A review on spectrophotometric methods for measuring the monophenolase and diphenolase activities of tyrosinase. J Agric Food Chem 55:9739–9749
Espín JC, García-Ruiz PA, Tudela J, Varón R, García-Cánovas F (1998) Monophenolase and diphenolase reaction mechanisms of apple and pear polyphenol oxidases. J Agric Food Chem 46:2968–2975
Rodriguez-Lopez JN, Fenoll LG, Garcia-Ruiz PA, Varon R, Tudela J, Thorneley RNF, Garcia-Canovas F (2000) Stopped-flow and steady-state study of the diphenolase activity of mushroom tyrosinase. Biochemistry 39:10497–10506
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Garcia-Sevilla F, Garrido-del Solo C, Duggleby RG, Garcia-Canovas F, Peyro R, Varon R (2000) Use of a windows program for simulation of the progress curves of reactants and intermediates involved in enzyme-catalyzed reactions. Biosystems 54:151–164
Schrödinger L (2010) The PyMOL molecular graphics system, 1.5.0.1. LLC
MOPAC2016. Stewart computational chemistry. Colorado Springs, 2016
Stewart JJP (2013) Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters. J Mol Model 19:1–32
Ismaya WT, Rozeboom HJ, Weijn A, Mes JJ, Fusetti F, Wichers HJ, Dijkstra BW (2011) Crystal structure of Agaricus bisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochemistry 50:5477–5486
Maria-Solano MA, Ortiz-Ruiz CV, Munoz-Munoz JL, Teruel-Puche JA, Berna J, Garcia-Ruiz PA, Garcia-Canovas F (2016) Further insight into the pH effect on the catalysis of mushroom tyrosinase. J Mol Catal B-Enzym 125:6–15
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Sanner MF (1999) Python: a programming language for software integration and development. J Mol Graph Model 17:57–61
Huey R, Morris GM, Olson AJ, Goodsell DS (2007) A semiempirical free energy force field with charge-based desolvation. J Comput Chem 28:1145–1152
Lente G, Espenson JH (2004) A kinetic study of the early steps in the oxidation of chlorophenols by hydrogen peroxide catalyzed by a water-soluble iron(iii) porphyrin. New J Chem 28:847–852
Simon A, Ballai C, Lente G, Fabian I (2011) Structure-reactivity relationships and substituent effect additivity in the aqueous oxidation of chlorophenols by cerium(iv). New J Chem 35:235–241
Espin JC, Varon R, Fenoll LG, Gilabert MA, Garcia-Ruiz PA, Tudela J, Garcia-Canovas F (2000) Kinetic characterization of the substrate specificity and mechanism of mushroom tyrosinase. Eur J Biochem 267:1270–1279
Fenoll LG, Rodríguez-López José N, Varón R, García-Ruiz Pedro A, García-Cánovas F, Tudela J (2000) Action mechanism of tyrosinase on meta- and para-hydroxylated monophenols. J Biol Chem 381:313
Xu H, Wolf C (2009) Efficient copper-catalyzed coupling of aryl chlorides, bromides and iodides with aqueous ammonia. Chem Commun 21:3035–3037
Fountoulaki S, Daikopoulou V, Gkizis PL, Tamiolakis I, Armatas GS, Lykakis IN (2014) Mechanistic studies of the reduction of nitroarenes by NaBH4 or hydrosilanes catalyzed by supported gold nanoparticles. ACS Catal 4:3504–3511
Garcia-Jimenez A, Teruel-Puche JA, Ortiz-Ruiz CV, Berna J, Tudela J, Garcia-Canovas F (2016) 4-n-butylresorcinol, a depigmenting agent used in cosmetics, reacts with tyrosinase. IUBMB Life 68:663–672
Munoz-Munoz JL, Garcia-Molina MD, Garcia-Molina F, Berna J, Garcia-Ruiz PA, Garcia-Moreno M, Rodriguez-Lopez JN, Garcia-Canovas F (2013) Catalysis and inactivation of tyrosinase in its action on o-diphenols, o-aminophenols and o-phenylendiamines: potential use in industrial applications. J Mol Catal B 91:17–24
Toussaint O, Lerch K (1987) Catalytic-oxidation of 2-aminophenols and ortho-hydroxylation of aromatic-amines by tyrosinase. Biochemistry 26:8567–8571
Ortiz-Ruiz CV, Berna J, Rodriguez-Lopez JN, Tomas V, Garcia-Canovas F (2015) Tyrosinase-catalyzed hydroxylation of 4-hexylresorcinol, an antibrowning and depigmenting agent: a kinetic study. J Agric Food Chem 63:7032–7040
Acknowledgements
This work was partially supported by grants from several Spanish organizations: Projects 19545/PI/14, 19304/PI/14 and 19240/PI/14 (Fundación Seneca, CARM, Murcia, Spain); Projects SAF2013-48375-C2-1-R and CTQ2014-56887-P (MINECO, Madrid); Projects UMU15452 and UMU17766 (University of Murcia, Murcia); C.V. Ortiz-Ruiz has a MEC-FPU fellowship (AP2010-4300), A. Garcia-Jimenez has a FPU fellowship from University of Murcia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garcia-Jimenez, A., Teruel-Puche, J.A., Ortiz-Ruiz, C.V. et al. Study of the inhibition of 3-/4-aminoacetophenones on tyrosinase. Reac Kinet Mech Cat 120, 1–13 (2017). https://doi.org/10.1007/s11144-016-1106-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-1106-6