Abstract
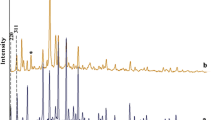
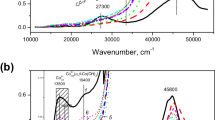
The hydroxylation of phenol was investigated using cobalt(II) complexes of bidentate ligands including N,N′-ethylenebis(salicylideneamine)(salen), N,N′-propylenbis(salicylideneamine)(salpn) and N,N′-phenylenebis(salicylideneamine)(salophen) encapsulated in zeolite-Y. The catalysts were characterized by Fourier transform infrared and X-ray diffraction analyses to confirm the complex encapsulation. These complexes catalyze the liquid-phase hydroxylation of phenol, through H2O2, to catechol and hydroquinone as major and minor products, respectively. The activities of all prepared catalysts were tested for the oxidation of phenol and hydrogen peroxide. The overall reaction conditions were optimized to get maximum hydroxylation gain to consider the concentration of substrate and oxidant, amount of catalyst, and the reaction temperature. The zeolite encapsulated complexes of cobalt(II) were found to be catalytically active toward the hydroxilation of phenol. Under the optimized reaction conditions, [Co-(salen)]-Y showed the highest conversion, about 43 %, after 6 h which was followed by [Co-(salpn)]-Y with 39 % conversion and [Co-(salophen)]-Y showing the lowest efficiency with 35 % conversion. The hydroxylation of phenol without catalyst and with Co/zeolite-Y catalyst showed poor results.







Similar content being viewed by others
References
Bowers C, Dutta PK (1990) J Catal 122:271–279
Knops-Gerrits P-P, De Vos D, Thibault-Starzyk F, Jacobs PA (1994) Nature 369:543–546
Agarwal DD, Bhatnagar RP, Jain R, Srivastava S (1990) J Chem Soc Perkin Trans 2:989–992
Maurya MR, Chandrakar AK, Chand S (2007) J Mol Catal A 274:192–201
Nethravathi BP, Reddy KR, Mahendra KN (2014) J Porous Mater 21:285–291
Maurya M, Saklani H, Kumar A, Chand S (2004) Catal Lett 93:121–127
Seelan S, Sinha AK (2003) Appl Cat A 238:201–209
Nethravathi B, Mahendra K (2010) J Porous Mater 17:107–113
Nethravathi BP, Mahendra KN, Reddy KR (2011) J Porous Mater 18:389–397
Sheldon RA, van Santen RA (1995) Catalytic oxidation: principles and applications: a course of the Netherlands Institute for Catalysis Research (NIOK). World Scientific, Singapore
Arpe HJ (1999) Industrial organic chemicals: starting materials and intermediates—An Ullmann’s Encyclopedia. Wiley, New York
Maurya MR, Titinchi SJJ, Chand S (2004) J Mol Catal A 214:257–264
Maurya M, Titinchi SJ, Chand S (2003) Catal Lett 89:219–227
Maurya M, Kumar M, Titinchi SJ, Abbo H, Chand S (2003) Catal Lett 86:97–105
Maurya MR, Titinchi SJJ, Chand S, Mishra IM (2002) J Mol Catal A 180:201–209
Jacob CR, Varkey SP, Ratnasamy P (1998) Microporous Mesoporous Mater 22:465–474
Maumy M, Capdevielle P (1996) J Mol Catal A 113:159–166
Bania KK, Bharali D, Viswanathan B, Deka RC (2012) Inorg Chem 51:1657–1674
Sophiphun O, Demir D, Föttinger K, Rupprechter G, Loiha S, Neramittagapong A, Prayoonpokarach S, Wittayakun J (2016) Reac Kinet Mech Cat 117:705–713
Sophiphun O, Föttinger K, Loiha S, Neramittagapong A, Prayoonpokarach S, Rupprechter G, Wittayakun J (2015) Reac Kinet Mech Cat 116:549–561
Peyrovi MH, Mahdavi V, Salehi MA, Mahmoodian R (2005) Catal Commun 6:476–479
Alizadeh M, Farzaneh F, Ghandi M (2003) J Mol Catal A 194:283–287
Güneş A, Bayraktar O, Yılmaz S (2006) Ind Eng Chem Res 45:54–61
Maurya MR, Titinchi SJJ, Chand S (2003) J Mol Catal A 193:165–176
Joseph T, Sajanikumari CS, Deshpande SS, Gopinathan S (1999) Indian J Chem Sect A 38:792–796
Hailu SL, Nair BU, Redi-Abshiro M, Aravindhan R, Diaz I, Tessema M (2015) RSC Adv 5:88636–88645
Hosseini-Ghazvini SMB, Safari P, Mobinikhaledi A, Zendehdel M (2015) Reac Kinet Mech Cat 115:703–718
Salavati-Niasari M, Bazarganipour M (2006) Catal Commun 7:336–343
Maurya MR, Singh B, Adão P, Avecilla F, Costa Pessoa J (2007) Eur J Inorg Chem 2007:5720–5734
Mobinikhaledi A, Zendehdel M, Safari P (2014) J Porous Mater 21:565–577
Mugo JN, Mapolie SF, van Wyk JL (2010) Inorg Chim Acta 363:2643–2651
Acknowledgments
The authors are grateful to the financial support from the Islamic Azad University, South Tehran branch. This study has been done under the format of project research “Zeolite-Y encapsulated metal complexes of Cobalt(II) as catalyst for the hydroxylation of phenol”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khodadadi, Z., Mahmoudian, R. Zeolite-Y encapsulated metal complexes of cobalt(II) as catalyst for the hydroxylation of phenol. Reac Kinet Mech Cat 119, 685–697 (2016). https://doi.org/10.1007/s11144-016-1072-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-1072-z