Abstract
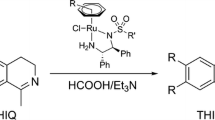
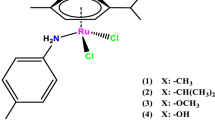
The role of the sulfonamide moiety of Noyori-Ikariya [Ru(II)Cl(η 6-p-cymene)(S,S)-(N-arylsulfonyl-DPEN)] (where DPEN = 1,2-diphenylethylene-1,2-diamine) half-sandwich complexes in the asymmetric transfer hydrogenation (ATH) of imines (1-methyl-3,4-dihydroisoquinoline and 6,7-dimethoxy-1-methyl-3,4-dihydroisoquinoline) was investigated. Nine complexes were synthesized and characterized, most of which have not been previously reported and a majority of the corresponding ligands (N-arylsulfonyl-DPEN) have not been described in imine ATH. The study demonstrates that the structure of the sulfonamide fragment strongly affects the catalytic activity. By monitoring the reaction kinetics, it was found that the reactivity of certain complexes was moderately enhanced and the enantioselectivity was affected as well, albeit to a lesser extent. No simple structure–activity pattern was found, suggesting that extensive screening experiments are necessary in order to obtain the optimal catalyst for a particular substrate. The study complements other previously reported works on structure–activity relationships concerning Ru(II)-catalyzed ATH by adding a new dimension of investigation.



Similar content being viewed by others
Notes
The reactions of [RuCl2(p-cymene)]2 with (S,S)-TsDPEN were also followed in situ by NMR spectroscopy, which confirmed full conversion to complexes 2. Hence, the rather low yields of complexes 2 were mainly due to their purification via crystallization on a small scale.
References
Davies NM, Teng XW (2003) Adv Pharm 1:242–252
Arnum PV (2006) Pharm Technol 30:58–67
Ojima I (2010) Catalytic Asymmetric synthesis, 3rd edn. Wiley, Hoboken
Knowles WS, Noyori R (2007) Acc Chem Res 40:1238–1239
Hashiguchi S, Fujii A, Takehara J, Ikariya T, Noyori R (1995) J Am Chem Soc 117:7562–7563
Fujii A, Hashiguchi S, Uematsu N, Ikariya T, Noyori R (1996) J Am Chem Soc 118:2521–2522
Uematsu N, Fujii A, Hashiguchi S, Ikariya T, Noyori R (1996) J Am Chem Soc 118:4916–4917
Wang C, Wu X, Xiao J (2008) Chem Asian J 3:1750–1770
Václavík J, Kačer P, Kuzma M, Červený L (2011) Molecules 16:5460–5495
Takehara J, Hashiguchi S, Fujii A, Inoue S, Ikariya T, Noyori R (1996) Chem Commun 2:233–234
Šot P, Vilhanová B, Pecháček J, Václavík J, Zápal J, Kuzma M, Kačer P (2014) Tetrahedron 25:1346–1351
Šot P, Kuzma M, Václavík J, Pecháček J, Přech J, Januščák J, Kačer P (2012) Organometallics 31:6496–6499
Václavík J, Pecháček J, Vilhanová B, Šot P, Januščák J, Matoušek V, Přech J, Bártová S, Kuzma M, Kačer P (2013) Catal Lett 143:555–562
Přech J, Václavík J, Šot P, Pecháček J, Vilhanová B, Januščák J, Syslová K, Pažout R, Maixner J, Zápal J, Kuzma M, Kačer P (2013) Catal Commun 36:67–70
Gulamhussen AM, Kačer P, Přech J, Kuzma M, Červený L (2009) React Kinet Catal Lett 97:335–340
Pecháček J, Václavík J, Přech J, Šot P, Januščák J, Vilhanová B, Vavřík J, Kuzma M, Kačer P (2013) Tetrahedron 24:233–239
Kuzma M, Václavík J, Novák P, Přech J, Januščák J, Červený J, Pecháček J, Šot P, Vilhanová B, Matoušek V, Goncharova II, Urbanová M, Kačer P (2013) Dalton Trans 42:5174–5182
Martins JED, Clarkson GJ, Wills M (2009) Org Lett 11:847–850
Martins JED, Contreras Redondo MA, Wills M (2010) Tetrahedron 21:2258–2264
Kei F, Cheung K, Hayes AM, Hannedouche J, Yim ASY, Wills M (2005) J Org Chem 70:3188–3197
Morris DJ, Hayes AM, Wills M (2006) J Org Chem 71:7035–7044
Martins JED, Wills M (2009) Tetrahedron 65:5782–5786
Hodgkinson R, Jurčík V, Zanotti-Gerosa A, Nedden HG, Blackaby A, Clarkson GJ, Wills M (2014) Organometallics 33:5517–5524
Ohkuma T, Utsumi N, Watanabe M, Tsutsumi K, Arai N, Murata K (2007) Org Lett 9:2565–2567
Shirai S, Nara H, Kayaki Y, Ikariya T (2009) Organometallics 28:802–809
Wang Z-J, Zhou H-F, Wang T-L, He Y-M, Fan Q-H (2009) Green Chem 11:767–769
Li X, Blacker J, Houson I, Wu X, Xiao J (2006) Synlett 8:1155–1160
Yin L, Zheng Y, Jia X, Li X, Chan ASC (2010) Tetrahedron 21:2390–2393
Lu C, Luo Z, Huang L, Li X (2011) Tetrahedron 22:722–727
Luo Z, Qin F, Yan S, Li X (2012) Tetrahedron 23:333–338
Přech J, Matoušek V, Václavík J, Pecháček J, Syslová K, Šot P, Januščák J, Vilhanová B, Kuzma M, Kačer P (2013) Am J Anal Chem 4:125–133
Acknowledgments
This work was financially supported by the Czech Science Foundation (P106/12/1276 and 15-08992S), grant for long-term conceptual development of the Institute of Microbiology of the Czech Academy of Sciences (RVO: 61388971) and the National Program of Sustainability (NPU I LO1215 and NPU I LO1509). The research was conducted within the infrastructure built up from the support of the Operational Program Prague—Competitiveness (projects CZ.2.16/3.1.00/22197, CZ.2.16/3.1.00/24501 and CZ.2.16/3.1.00/24023).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matuška, O., Zápal, J., Hrdličková, R. et al. Role of the sulfonamide moiety of Ru(II) half-sandwich complexes in the asymmetric transfer hydrogenation of 3,4-dihydroisoquinolines. Reac Kinet Mech Cat 118, 215–222 (2016). https://doi.org/10.1007/s11144-016-0991-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-0991-z