Abstract
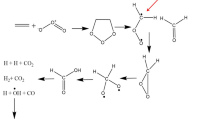
The effects of temperature, the concentrations of methanol and the trans-2,3-bis(diphenylphosphinomethyl)norbornane (TBDPN) promoting additive on the rate of cyclohexene hydrocarbomethoxylation catalyzed by the Pd(OAc)2/p-toluenesulfonic acid system were studied. It was found that in the 358–383 K temperature range, the increase in the CH3OH concentration from 0 to 0.15 mol/L induces a virtually linear increase in the reaction rate, which slows down as the methanol concentration further increases. In the temperature range of 343–373 K, the dependences of the reaction rate on the TBDPN concentration pass through maxima at [TBDPN] = (3.0–4.0) × 10−3 mol/L. The results were interpreted in terms of the hydride mechanism that included diphosphinepalladium complexes as intermediates and was supplemented by ligand exchange reactions, resulting in a decrease in the activity of the palladium catalyst. The effective constants of the previously derived kinetic equation in the temperature range of 343–383 K were estimated by the least-squares method. The effective activation energies were determined and used to evaluate the enthalpy change in the ligand exchange reaction between the complexes Pd(TBDPN)3 and [HPd(TBDPN)(CH3OH)]OTs. The zerovalent complex Pd(TBDPN)3 was concluded to be more stable than the hydride complex [HPd(TBDPN)(CH3OH)]OTs.






Similar content being viewed by others
References
Kiss G (2001) Chem Rev 101:3435–3456
Cavinato G, Vavasori A, Toniolo L, Dolmella A (2004) Inorg Chimica Acta 357:2737–2747
Guiu E, Caporali M, Muñoz B, Müller C, Lutz M, Spek AL, Claver C, van Leeuwen PWNM (2006) Organometallics 25:3102–3104
Kron TE, Terekhova MI, Noskov YUG, Petrov ES (2001) Kinet Catal 42:182–189
Nomura SOM, Aiko T, Inoue Y (1997) J Mol Catal A: Chem 115:289–295
Drent E, Buzelaar PHM (1996) Chem Rev 96:663–681
Nifant’ev IE, Sevostyanova NT, Averyanov VA, Batashev SA, Vorobiev AA, Toloraya SA, Bagrov VV, Tavtorkin AN (2012) Appl Catal A General 449:145–152
Yoshida H, Sugita N, Kudo K, Takezaki Y (1976) Bull Chem Soc Jpn 49:2245–2249
Vavasori A, Toniolo L, Cavinato G (2003) J Mol Catal A: Chem 191:9–21
Aver’yanov VA, Batashev SA, Sevost’yanova NT, Nosova NM (2006) Kinet Catal 47:375–382
Aver’yanov VA, Sevost’yanova NT, Batashev SA, Demerlii AM (2013) Petrol Chem 53:39–45
Aver’yanov VA, Sevost’yanova NT, Batashev SA, Nesolenaya SV (2006) Petrol Chem 46:405–414
Aver’yanov VA, Sevost’yanova NT, Batashev SA (2008) Petrol Chem 48:287–295
Averyanov VA, Sevostyanova NT, Batashev SA, Vorobiev AA, Rodionova AS (2014) Russ J Phys Chem B 8:140–147
Sevostyanova NT, Batashev SA, Averyanov VA, Demerley AM (2012) Petrol Chem 52:35–40
Nifant’ev IE, Batashev SA, Toloraya SA, Tavtorkin AN, Sevostyanova NT, Vorobiev AA, Bagrov VV, Averyanov VA (2011) J Mol Catal A: Chem 350:64–68
Seayad A, Kelkar AA, Toniolo L, Chaudhari RV (2000) J Mol Catal A: Chem 151:47–59
Vavasori A, Cavinato G, Toniolo L (2001) J Mol Catal A: Chem 176:11–18
Terekhova MI, Sigalov AB, Petrova NE, Petrov ES (1985) J Gen Chem USSR 55:944–945
Verspui G, Moiseev I, Sheldon RA (1990) J Organomet Chem 586:196–199
Noskov YuG, Simonov AI, Petrov ES (2001) Kinet Catal 42:182–189
Seayad A, Jayasree S, Damodaran K, Toniolo L, Chaudhari RV (2000) J Organomet Chem 601:100–107
Terekhova MI, Petrova NE, Shifrina RR, Petrov ES (1988) Russ J Gen Chem 58:658–661
Cavinato G, Toniolo L, Vavasori A (2004) J Mol Catal A: Chem 219:233–240
Bardi R, Piazzasi AM, Cavinato G, Toniolo L (1985) Inorg Chim Acta 102:99–103
Bardi R, Del Pra A, Piazzasi AM, Toniolo L (1979) Inorg Chim Acta 35:L345–L346
Cavinato G, Toniolo L (1979) J Mol Catal A: Chem 6:111–122
Petrov ES, Noskov YUS (1998) Ross Khim Zh 42:149–157
Acknowledgments
This study was supported by the Russian Foundation for Basic Research within the framework of Projects No. 14-08-00535.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nifant’ev, I., Sevostyanova, N., Batashev, S. et al. Kinetic aspects of the influence of concentrations of methanol and the trans-2,3-bis(diphenylphosphinomethyl)norbornane promoting additive on the hydrocarbomethoxylation of cyclohexene catalyzed by the Pd(OAc)2/p-toluenesulfonic acid system. Reac Kinet Mech Cat 116, 63–77 (2015). https://doi.org/10.1007/s11144-015-0888-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-015-0888-2