Abstract
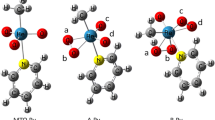
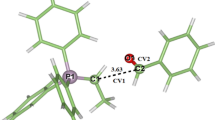
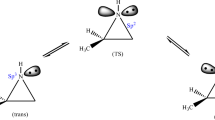
Recently, the work focusing on the role of alkyl chain length in the N[R,222][Tf2N] ionic liquids and its reflection in the kinetic parameters of stereoselective hydrogenation of methylacetoacetate over (R)-[RuCl(binap)(p-cymene)]Cl complex was reported. Irregular trends in the principle parameter of enantioselectivity were observed. Here, a possible theoretical explanation of such irregular trends elucidated with the help of molecular simulations methods is presented. A stepwise approach is proposed for evaluating the energetically most stable conformers of a series of individual N[R,222][Tf2N] for the interpretation of selectivity-structure effects observed experimentally. Initially, Monte Carlo molecular mechanics was used followed by a semi-empirical PM3 method to elucidate also the characteristic thermodynamic functions of state and theoretical molecular spectra. The density functional theory was finally applied. The specific absolute entropy data revealed that due to the partition of the cation and anion twisted closer approach the ionic liquids N[8,222][Tf2N] and N[12,222][Tf2N] may stabilize the structures of the (R)-[RuCl(binap)(p-cymene)]Cl complex.












Similar content being viewed by others
References
Noyori R (2003) Adv Synth Catal 345:15
McDonald AR, Muller C, Voght D (2008) Green Chem 10:424
Blaser HU, Jallet HP, Spindler F (1996) J Mol Catal A 107:85
Bartek L, Kluson P, Cerveny L (2005) Chem Listy 99:157
Jahjah M, Alame M, Pellet-Rostaing P, Lemaire M (2007) Tetrahedron Asymm 19:2305
Wolfson A, Vankelecom IFJ, Geresh S, Jacobs PA (2004) J Mol Catal A 217:21
Zsigmond A, Notheisz F, Kluson P, Floris T (2010) In: Barbaro P, Liguori F (eds) Heterogenized homogeneous catalysts for fine chemicals production—catalysis by metal complexes, vol 34. Springer, Berlin
Floris T, Kluson P, Muldoon MJ, Pelantova H (2010) Catal Let 134:279
Floris T, Kluson P, Pelantova H, Bartek L (2009) Appl Catal A 366:160
Cerna I, Kluson P, Bendova M, Pekarek T, Floris T, Pelantova H (2011) Chem Eng Proc 50:264
Floris T, Kluson P, Slater M (2011) React Kinet Catal Mech 102:67
Hallett JP, Welton T (2011) Chem Rev 111:3508
Olivier-Bourbigou H, Magna L, Morvan D (2010) Appl Catal A 373:1
Picquet M, Poinsot D, Stutzmann S, Tkatchenko I, Tommasi I, Wasserscheid P, Zimmermann J (2004) Topics Catal 29:139
Wasserscheid P, Keim W (2000) Angewandte Chem Int Ed 39:3772
Frisch AC, Webb PB, Zhao G, Muldoon MJ, Pogorzelec PJ, Cole-Hamilton DJ (2007) Dalton Trans 47:5531
Jutz F, Andanson JM, Baiker A (2009) J Catal 268:356
Allouche AR (2011) J Comp Chem 32(174):182
Halgren TA (1996) J Comp Chem 490:519
Hehre WJ (2003) A guide to molecular mechanics and quantum chemical calculations. Wavefunction Inc, Irvine
Young D (2001) Computational chemistry. Wiley–Interscience, New York, p 330
Frisch MJ et al (2003) Gaussian 03. Gaussian Inc, Wallingford
Young D (2001) Computational chemistry. Wiley–Interscience, New York, p 336
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, Oxford
Johansson KM, Izgorodina EI, Forsyth M, MacFarlane DR, Seddon KR (2008) Phys Chem Chem Phys 10:2972
Hanke CG, Price SL, Lynden-Bell RM (2001) Mol Phys 99:801
Bhargava BL, Balasubramanian S (2008) J Phys Chem B 112:7566
Wang Y, Voth GA (2005) J Am Chem Soc 127:12192
Bhargava BL, Klein ML (2009) Soft Matter 5:3475
Berg RW (2007) Monatshefte Chem 138:1045
Costa Gomes MF, Canongia Lopes JN, Padua AAH (2009) In: Kirchner B (ed) Ionic liquids—topics in current chemistry, vol 290. Springer, Berlin
Kirchner B (2009) In: Kirchner B (ed) Ionic liquids—topics in current chemistry, vol 290. Springer, Berlin
Acknowledgments
Authors gratefully acknowledge the financial contribution of the Ministry of Industry and Trade of the Czech Republic, Project Grant No. FR-TI3/057. Academy of Sciences of the Czech Republic is also cordially acknowledged for supporting the bilateral CZ-UK cooperation project with Bangor University, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dytrych, P., Kluson, P., Slater, M. et al. Theoretical interpretation of the role of the ionic liquid phase in the (R)-Ru-BINAP catalyzed hydrogenation of methylacetoacetate. Reac Kinet Mech Cat 111, 475–487 (2014). https://doi.org/10.1007/s11144-013-0659-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0659-x