Abstract
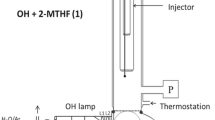
Second generation (2G) biofuels are produced from non-edible lignocellulosic biomass providing sustainable alternatives for fossil fuels. The OH reaction rate constant has been determined for the 2G biofuel ethyl levulinate (CH3C(O)CH2CH2C(O)OCH2CH3, ELA) (1) for the first time. The direct reaction kinetic technique of pulsed laser photolysis (PLP) coupled with resonance fluorescence (RF) detection of OH radicals has been used to determine the rate constant value of k 1 = (3.43 ± 0.36) × 10−12 cm3 molecule−1 s−1 (298 K), where the error given designates 2σ accuracy. Implications for atmospheric chemistry have been discussed.

Similar content being viewed by others
References
Tollefson J (2008) Nature 452:670
Hayes D (2009) Catal Today 145:138
Fitzpatrick SW (1997) Production of levulinic acid from carbohydrate containing materials. US Patent 5,608,105, Assignee: Biofine Incorporated
Horváth I, Mehdi H, Fábos V, Boda L, Mika L (2008) Green Chem 10:238
Farkas M, Szabó E, Zügner GL, Zsibrita D, Illés Á, Petri B, Dóbé S (2011) Kinetic studies of second generation biofuels. Proceedings of the European combustion meeting, Paper no. 310, University of Cardiff, Cardiff
Hayes DJ, Fitzpatrick SW, Ross JRH, Kamm M (2005) Biorefineries: industrial processes and products. Wiley, Weinheim
Zügner GL (2011) Climate change and atmospheric chemistry: kinetic studies of elementary chemical and photochemical reactions. PhD Thesis, Budapest University of Technology and Economics (submitted)
Sander SP, Abbatt J, Barker JR, Burkholder JB, Friedl RR, Golden DM, Huie RE, Kolb CE, Kurylo MJ, Moortgat GK, Orkin VL, Wine PH (2011) Chemical kinetics and photochemical data for use in atmospheric studies. Evaluation no. 17, JPL Publication 10-6, Jet Propulsion Laboratory, Pasadena
Kwok E, Atkinson R (1995) Atmos Environ 29:1685
Farkas M (2011) Unpublished work. Chemical Research Center, Budapest
Heard DE, Pilling MJ (2003) Chem Rev 29:5163
Acknowledgment
This work has been supported by the Hungarian Scientific Research Fund OTKA (Contract OMFB-00991/2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farkas, M., Illés, Á., Petri, B. et al. Direct rate constant for the reaction of OH radicals with the biofuel molecule ethyl levulinate. Reac Kinet Mech Cat 104, 251–257 (2011). https://doi.org/10.1007/s11144-011-0364-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-011-0364-6