Abstract
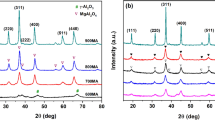
Alumina-supported cobalt metal catalysts are studied in the preparation of 1-phenylethanol by the selective hydrogenation of acetophenone. X-ray diffraction was used to investigate the phase of the catalysts. Effects of reaction temperatures, Co loading, and catalysts preparation method on the activity of the hydrogenation of acetophenone and the selectivity of 1-phenylethanol were studied. The results show that the preparation method and Co loading influence the dispersion of the catalyst. The hydrogenation of carbonyl groups has priority to hydrogenation of phenyl groups over alumina-supported Co catalysts. The selectivity of 1-phenylethanol decreases when the reaction temperature is increased under the same conversion conditions. With the Co loading increasing, the reaction activity increases while the selectivity of 1-phenylethanol decreases. The selectivity of 1-phenylethanol reaches 98% when the conversion is up to 98.2% at 373 K over the catalyst prepared by impregnation.





Similar content being viewed by others
References
Reddy BM, Rao KN, Reddy GK (2009) Catal Lett 131(1–2):328
Liu HP, Lu GZ, Guo Y et al (2009) Catal Commun 10(9):1324
Quintanilla A, Bakker JJW, Kreutzer MT et al (2008) J Catal 257(1):55
Chen CS, Chen HW (2004) Appl Catal A 260(2):207
Ji YL, Ma XB, Wu XJ et al (2007) Appl Catal A 332(2):247
Chen CS, Chen HW, Cheng WH (2003) Appl Catal A 248(1–2):117
Casagrande M, Storaro L, Talon A et al (2002) J Mol Catal A 188(1–2):133
Hamarthibault S, Masson J, Fouilloux P et al (1993) Appl Catal A 99(2):131
Masson J, Vidal S, Cividino P et al (1993) Appl Catal A 99(2):147
Bertero NM, Apesteguia CR, Marchi AJ (2008) Appl Catal A 349(1–2):100
Chen XF, Li HX, Dai WL et al (2003) Appl Catal A 253(2):359
Li HX, Chen XF, Wang MH et al (2002) Appl Catal A 225(1–2):117
Jiang L, Zhu YF, Xiang YZ et al (2007) Chin J Catal 28(03):281
Acknowledgments
The work was supported by Excellent Young Teachers funding schemes of Zhe-jiang Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X.B. Preparation of 1-phenylethanol by selective hydrogenation of acetophenone over alumina-supported Co catalysts. Reac Kinet Mech Cat 102, 417–424 (2011). https://doi.org/10.1007/s11144-010-0268-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-010-0268-x