Abstract
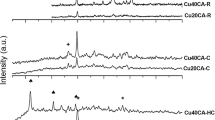
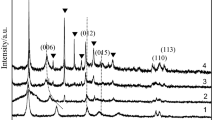
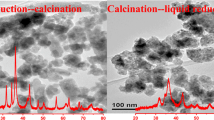
The activities of the Cu–Cr–Mg–Al catalysts in the dehydrogenation of cyclohexanol to cyclohexanone were correlated with particle sizes and the reducibility of the copper species based on XRD and TPR characterizations. Cu0 was proven to be the active center of the catalysts calcined at low temperatures and some solid solution (MgCu2O3) and spinel (CuAl2O4 or MgAl2O4) were produced when the calcination temperature reached 1,000 °C. The Cu–Cr–Mg–Al catalyst prepared by the co-precipitation method and calcined at 400 °C showed the best activity, with a conversion of 79.7% and a selectivity of 98.6% at 300 °C.






Similar content being viewed by others
References
Nagaraja BM, Padmasri AH, Seetharamulu P, Reddy KHP, Raju, BD, Rao KSR (2007) J Mol Catal A Chem 278:29
Zheng HY, Zhu YL, Huang L, Zeng ZY, Wan HJ, Li YW (2008) Catal Commun 9:342
Fridman VZ, Davydov AA (2000) J Catal 195:20
Zhu WC, Wang LX, Liu SY, Wang ZL (2008) React Kinet Catal Lett 93:93
Ji DH, Zhu WC, Wang ZL, Wang GJ (2007) Catal Commun 8:1891
Fridman VZ, Davydov AA, Titievsky K (2004) J Catal 222:545
Nagaraja BM, Kumar VS, Shashikala V, Padmasri AH, Reddy SS, Raju BD, Rao KSR (2004) J Mol Catal A Chem 223:339
Bai GY, Wang HL, Ning HS, He F, Chen GF (2008) React Kinet Catal Lett 94:375
Ji DH, Zou XJ, Zheng DF, Wang LX, Liu SY, Zhu WC, Wang GJ, Wang ZL (2008) Pol J Chem 82:1097
Ramaswamy V, Malwadkar S, Chilukuri S (2008) Appl Catal B Environ 84:21
Yahiro H, Nakaya K, Yamamoto T, Saiki K, Yamaura H (2006) Catal Commun 7:228
Yang J, Zheng HY, Zhu YL, Zhao GW, Zhang CH, Teng BT, Xiang HW, Li YW (2004) Catal Commun 5:505
Dow WP, Wang YP, Huang TJ (1996) J Catal 160:155
Bai GY, Chen LG, Li Y, Yan XL, He F, Xing P, Zeng T (2004) Appl Catal A Gen 277:253
Bai GY, Li Y, Yan XL, He F, Chen LG (2004) React Kinet Catal Lett 82:33
Acknowledgements
The authors thank Professor David Knight for his helpful discussions. Financial support by the National Natural Science Foundation of China (20806018), the Natural Science Foundation of Hebei Province (B2007000156) and the Science Project of the Hebei Education Department (2005350) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, G., Fan, X., Wang, H. et al. Effects of the preparation method and calcination temperature on Cu–Cr–Mg–Al catalysts for the dehydrogenation of cyclohexanol. React Kinet Catal Lett 98, 341–348 (2009). https://doi.org/10.1007/s11144-009-0082-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-009-0082-5