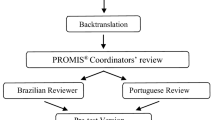
Abstract
Objective
This paper goal was to validate the Portuguese version (Brazil/Portugal) of the Anger, Anxiety, and Depressive Symptoms item banks of the Pediatric PROMIS® Emotional Distress domain (version 1.0) for the Brazilian and Portuguese pediatric population.
Method
The total of 1216 participants answered a self-applied version of the Portuguese Anger, Anxiety, and Depressive Symptoms item banks. Reliability was assessed through internal consistency, test–retest reliability, and total information curve (TIC). Confirmatory Factor Analysis (CFA) with a bifactor model was used to confirm construct validity and IRT assumptions. Item calibration was performed according to Graded Response Model (GRM). Differential Item Functioning (DIF) was analyzed according the participants’ age, gender, health condition (healthy versus chronic disease), and language.
Results
Internal consistency reliability (Cronbach's alpha coefficient = 0.84) and test–retest reliability (intraclass correlation = 0.93) were accurate. Unidimensionality, Local Independency, and construct validity were verified by CFA (CFI = 0.93; TLI = 0.93; RMSEA = 0.05; χ2 = 3052.4 with DIF = 557 and P value = 0.595). GRM was adjusted, and Emotional Distress had a satisfactory coverage. DIF was not significant.
Conclusion
The results obtained indicate the adequacy of the psychometric properties of the Portuguese version (Brazil/Portugal) of the Anger, Anxiety, and Depressive Symptoms item banks of the Pediatric PROMIS® Emotional Distress domain.




Similar content being viewed by others
References
Promis Cooperative Group. (2008). Unpublished manual for the Patient-Reported Outcomes Measurement Information System (PROMIS) (Version 1.1). https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.169.5144&rep=rep1&type=pdf. Accessed 10 May 2017.
Irwin, D. E., Stucky, B., Langer, M. M., Thissen, D., DeWitt, E. M., Lai, J.-S., et al. (2010). An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Quality of Life Research,19(4), 595–607.
Irwin, D. E., Stucky, B. D., Langer, M. M., Thissen, D., DeWitt, E. M., Lai, J.-S., et al. (2012). PROMIS Pediatric Anger Scale: An item response theory analysis. Quality of Life Research,21(4), 697–706.
Ader, D. N. (2007). Developing the patient-reported outcomes measurement information system (PROMIS). Medical Care,45(5 Suppl. 1), S1–S2.
Guyatt, G. H., Feeny, D. H., & Patrick, D. L. (1993). Measuring health-related quality of life. Annals of Internal Medicine,118(8), 622–629.
Baldwin, W. (2000). Information no one else knows: The value of self-report. In A. A. Stone, et al. (Eds.), The Science of Self-Report (pp. 3–7). London: LEA.
Riley, W. T., Pilkonis, P. A., & Cella, D. (2011). Application of the National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS®) to Mental Health Research. The Journal of Mental Health Policy and Economics,14(4), 201–208.
Katon, W. J. (2003). Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Society of Biological Psychiatry,54(3), 216–226.
American Psychiatric Association. (2014). DSM-5: Manual Diagnóstico e Estatístico de Transtornos Mentais (5th ed.). Porto Alegre: Artmed.
Bayer, J. K., Rapee, R. M., Hiscock, H., Ukoumunne, O. C., Mihalopoulos, C., & Wake, M. (2011). Translational research to prevent internalizing problems early in childhood. Depression and Anxiety,28(1), 50–57.
Woodward, L. J., & Fergusson, D. M. (2001). Life course outcomes of young people with anxiety disorders in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry,40(9), 1086–1093.
Fleischmann, A., Bertolote, J. M., Belfer, M., & Beautrais, A. (2005). Completed suicide and psychiatric diagnoses in young people: A critical examination of the evidence. American Journal of Orthopsychiatry,75(4), 676–683.
Cella, D., Gershon, R., Lai, J. S., & Choi, S. (2007). The future of outcomes measurement: Item banking, tailored shortforms, and computerized adaptive assessment. Quality of Life Research,16(Suppl. 1), 133–141.
Reeve, B. B., Hays, R. D., Bjorner, J. B., Cook, K. F., Crane, P. K., Teresi, J. A., et al. (2007). Psychometric evaluation and calibration of health-related quality of life item banks: Plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Medical Care,45(5 Suppl. 1), 22–31.
Pinto, M. N. F. de C. (2014). Tradução e adaptação cultural dos bancos de itens dos domínios dificuldades emocionais e aspectos sociais do pediatric Patient-Reported Outcomes Measurement Information System - PROMIS pediatric® (versão 1.0) para a língua portuguesa. Master’s Thesis, Federal University of Uberlândia, Uberlândia.
Eremenco, S. L., Cella, D., & Arnold, B. J. (2005). A comprehensive method for the Translation and Cross-Cultural Validation of Health status questionnaire. Evaluation and the Health Professions, Baltimore,8(2), 212–232.
Mokkink, L. B., Terwee, C. B., Patrick, D. L., Alonso, J., Stratford, P. W., Knol, D. L., et al. (2010). The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation,19(4), 539–549. https://doi.org/10.1007/s11136-010-9606-8.
García-Pérez, M. A. (2017). An analysis of (dis)ordered categories, thresholds, and crossings in difference and divide-by-total IRT models for ordered responses. The Spanish Journal of Psychology,20, E10.
De Vet, H. C., Terwee, C. B., Mokkink, L. B., & Knol, D. L. (2011). Measurement in medicine. Cambridge: Cambridge University.
McHorney, C. A., et al. (1994). The MOS 36 item short-form healthy survey (SF-36): III. Test of data quality, scaling assumptions and reliability across diverse patient groups. Medical Care,32(1), 40–66.
Cronbach, L. J. (1951). Coefficient alpha and the internal structure of tests. Psychometrika,16(3), 297–334.
Walter, S. D., Eliasziw, M., & Donner, A. (1998). Sample size and optimal designs for reliability studies. Statistics in Medicine,17(1), 101–110. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1%3c101:aid-sim727%3e3.0.co;2-e.
Winer, B. J., Brown, D. R., & Michels, K. M. (1991). Statistical principles in experimental design (3rd ed.). New York: MacGraw-Hill.
Qin, S., Nelson, L., McLeod, L., Eremenco, S., & Coons, S. J. (2019). Assessing test-retest reliability of patient-reported outcome measures using intraclass correlation coefficients: Recommendations for selecting and documenting the analytical formula. Quality of Life Research,28(4), 1029–1033.
Weir, J. P. (2005). Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. Journal of Strength and conditioning Research,19(1), 231–240.
Terwee, C. B., Bot, S. D., Boer, M. R., van der Windt, D. A. W. M., Knol, D. L., Dekker, J., et al. (2007). Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology,60(1), 34–42.
Yuan, K. H. (2005). Fit indices versus test statistics. Multivariate Behavioral Research,40, 115–148.
Kline, R. B. (2005). Principles and practice of structural equation modeling (2nd ed.). New York: The Guilford Press.
Hair, J. F., Anderson, R. E., Tatham, R. L., & Black, W. C. (2006). Multivariate data analysis. New Jersey: Prentice Hall.
Tabachnick, B. G., & Fidell, L. S. (1996). Using multivariate statistics. New York: Harper Collins.
Samejima, F. (1969). Estimation of latent ability using a response pattern of graded scores. Psychometrika Monograph RB-68-2. Princenton: Educational Testing Service.
Thissen, D., Chen, W. H., & Bock, R. D. (2003). Multilog (version 7). Lincolnwood, IL: Scientifics Software International.
Hambleton, R. K., Swaminathan, H., & Rogers, W. H. (1991). Fundamentals of item response theory. Newbury Park: Sage.
Baker, F. B. (2001). The basics of item response theory (2nd ed.). Washington, DC: Eric Clearinghouse on Assessment and Evaluation.
Edelen, M. O., & Reeve, B. B. (2001). Applying item response theory (IRT) modeling to questionnaire development, evaluation, and refinement. Quality of Life Research,16(Suppl. 1), 5–18.
Choi, S. W., Gibbons, L. E., & Crane, P. K. (2011). Iordif: An R package for detecting differential item functioning using iterative hybrid ordinal logistic regression/item response theory and Monte Carlo simulations. Journal of Statistical Software,39(8), 1–30.
Zumbo, B. D. (1999). A handbook on the theory and methods of differential item functioning (DIF): Logistic regression modeling as a unitary framework for binary and liket-type (ordinal) item scores. Ottawa: Directorate of Human Resources Research and Evaluation, Department of National Defense.
Fayers, P. M., & Machin, D. (2007). Quality of life. Chichester: Wiley.
Harrison, D. A. (1986). Robustness of IRT parameter estimation to violations of the unidimensionality assumption. Journal of Educational and Behavioral Statistics,11, 91–115.
Toland, M. D. (2013). Practical guide to conducting an item response theory analysis. The Journal of Early Adolescence,34(1), 120–151. https://doi.org/10.1177/027243161351133.
Polanczyk, G. V., Salum, G. A., Sugaya, L. S., Caye, A., & Rohde, L. A. (2015). Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of Child Psychology and Psychiatry,56(3), 345–365.
Acknowledgements
The authors would like to thank the PROMIS® team for their collaboration and technical assistance in the transcultural adaptation process, in particular, the researchers of the Medical Social Science Department at Northwestern University (Chicago—USA) David Cella, PhD and Helena Correia and to FACITtrans director, Benjamin Arnold (Elmhurst, USA). The authors also thank the State of Minas Gerais Foundation for Research Support (FAPEMIG) for the financial support and the Quality of Life research group of the Faculty of Medicine at the Federal University of Uberlândia (FAMED-UFU).
Funding
This study was funded by the State of Minas Gerais Foundation for Research Support (Fapemig) (PPM-00306–08).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pinto, M.N.F.C., Pinto, R.M.C., Mendonça, T.M.S. et al. Validation and calibration of the patient-reported outcomes measurement information system: Pediatric PROMIS® Emotional Distress domain item banks, Portuguese version (Brazil/Portugal). Qual Life Res 29, 1987–1997 (2020). https://doi.org/10.1007/s11136-020-02447-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-020-02447-z