Abstract
Purpose
The EuroQol five-dimension questionnaire (EQ-5D) is the most commonly used instrument to obtain utility values for cost-effectiveness analyses of treatments for Crohn’s disease (CD). We aimed to compare the measurement properties of the two adult versions of EQ-5D (EQ-5D-3L and EQ-5D-5L) in patients with CD.
Methods
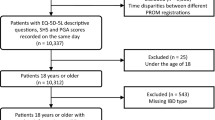
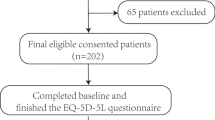
Between 2016 and 2017, a multicentre cross-sectional survey was carried out. Consecutive outpatients with CD completed the 3L, 5L and EQ visual analogue scale (VAS). Disease severity was graded by the Crohn’s Disease Activity Index (CDAI) and Perianal Disease Activity Index (PDAI). The 3L and 5L were compared in terms of feasibility, agreement, ceiling effect, redistribution properties, discriminatory power, convergent and known-groups validity.
Results
Two-hundred and six patients (54.9% male, mean age 35 ± 11 years) participated in the survey. For 3L, 25 unique health states were observed versus 59 for the 5L. The overall ceiling effect decreased from 29.6% (3L) to 25.5% (5L). Absolute discriminatory power improved (mean Shannon index 0.84 vs. 1.18). The 3L correlated stronger with EQ VAS and CDAI scores, whereas the 5L with PDAI. The 5L demonstrated a better known-groups validity on the basis of age, perianal fistulas, extraintestinal manifestations and disability.
Conclusions
This is the first study to report the impact of CD on quality of life using the EQ-5D-5L questionnaire. The 5L seems to perform better than 3L in terms of feasibility, ceiling effect, discriminatory power and known-groups validity. Understanding the differences in psychometrics between the 3L and 5L is essential as they have substantial implications for financial decision-making about CD treatments.


Similar content being viewed by others
Change history
03 December 2020
The correct ORCID id is linked to the author Tamás Molnár
References
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2018). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. The Lancet, 390(10114), 2769–2778.
Kaplan, G. G. (2015). The global burden of IBD: From 2015 to 2025. Nature Reviews Gastroenterology & Hepatology, 12(12), 720–727.
Torres, J., Mehandru, S., Colombel, J. F., & Peyrin-Biroulet, L. (2017). Crohn’s disease. The Lancet, 389(10080), 1741–1755.
Harbord, M., Annese, V., Vavricka, S. R., Allez, M., Barreiro-de Acosta, M., Boberg, K. M., et al. (2016). The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. Journal of Crohn’s and Colitis, 10(3), 239–254.
van der Have, M., van der Aalst, K. S., Kaptein, A. A., Leenders, M., Siersema, P. D., Oldenburg, B., et al. (2014). Determinants of health-related quality of life in Crohn’s disease: A systematic review and meta-analysis. Journal of Crohn’s and Colitis, 8(2), 93–106.
Brodszky, V., Rencz, F., Pentek, M., Baji, P., Lakatos, P. L., & Gulacsi, L. (2016). A budget impact model for biosimilar infliximab in Crohn’s disease in Bulgaria, the Czech Republic, Hungary, Poland, Romania, and Slovakia. Expert Review of Pharmacoeconomics & Outcomes Research, 16(1), 119–125.
Rencz, F., Pentek, M., Bortlik, M., Zagorowicz, E., Hlavaty, T., Sliwczynski, A., et al. (2015). Biological therapy in inflammatory bowel diseases: Access in Central and Eastern Europe. World Journal of Gastroenterology, 21(6), 1728–1737.
Boncz, I., & Sebestyen, A. (2006). Financial deficits in the health services of the UK and Hungary. The Lancet, 368(9539), 917–918.
Gulacsi, L., Pentek, M., Rencz, F., Brodszky, V., Baji, P., Vegh, Z., et al. (2017). Biosimilars for the management of inflammatory bowel diseases: Economic considerations. Current Medicinal Chemistry. https://doi.org/10.2174/0929867324666170406112304
Haute Autorité de Santé (HAS). (2012). Choices in methods for economic evaluation. Saint-Denis La Plaine: Department of Economics and Public Health Assessment. Retrieved from 22 April 2018. https://www.has-sante.fr/portail/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf.
National Institute for Health and Care Excellence (NICE). (2013). Guide to the methods of technology appraisal. Retrieved from 01 April 2018. https://www.nice.org.uk/guidance/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
Zorginstituut Nederland. (2016). Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg. Diemen: Zorginstituut Nederland. Retrieved from 22 April 2018. https://www.zorginstituutnederland.nl/binaries/zinl/documenten/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg.pdf.
Cleemput, I., Neyt, M., Van de Sande, S., & Thiry, N. (2012). Belgian guidelines for economic evaluations and budget impact analyses, (2nd edn). Retrieved from 22 Jan 2017. https://kce.fgov.be/sites/default/files/atoms/files/KCE_183_economic_evaluations_second_edition_Report.pdf.
Rencz, F., Gulacsi, L., Drummond, M., Golicki, D., Prevolnik Rupel, V., Simon, J., et al. (2016). EQ-5D in central and eastern Europe: 2000–2015. Quality of Life Research, 25(11), 2693–2710.
Rowen, D., Azzabi Zouraq, I., Chevrou-Severac, H., & van Hout, B. (2017). International regulations and recommendations for utility data for health technology assessment. Pharmacoeconomics, 35(Suppl 1), 11–19.
Sanders, G. D., Neumann, P. J., Basu, A., Brock, D. W., Feeny, D., Krahn, M., et al. (2016). Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA, 316(10), 1093–1103.
Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies. (2006). Retrieved from 04 Dec 2017. https://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf.
Huoponen, S., & Blom, M. (2015). A systematic review of the cost-effectiveness of biologics for the treatment of inflammatory bowel diseases. PLoS ONE. 10(12), e0145087.
Pillai, N., Dusheiko, M., Burnand, B., & Pittet, V. (2017). A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLoS ONE. 12(10), e0185500.
Rencz, F., Gulacsi, L., Pentek, M., Gecse, K. B., Dignass, A., Halfvarson, J., et al. (2017). Cost-utility of biological treatment sequences for luminal Crohn’s disease in Europe. Expert Review of Pharmacoeconomics & Outcomes Research, 17(6), 597–606.
Punekar, Y. S., Sunderland, T., Hawkins, N., & Lindsay, J. (2010). Cost-effectiveness of scheduled maintenance treatment with infliximab for pediatric Crohn’s disease. Value in Health, 13(2), 188–195.
Baji, P., Gulácsi, L., Brodszky, V., Végh, Z., Danese, S., Irving, P. M., et al. (2018). Cost-effectiveness of biological treatment sequences for fistulising Crohn’s disease across Europe. United European Gastroenterology Journal, 6(2), 310–321.
Malinowski, K. P., & Kawalec, P. (2016). Health utility of patients with Crohn’s disease and ulcerative colitis: A systematic review and meta-analysis. Expert Review of Pharmacoeconomics & Outcomes Research, 16(4), 441–453.
Brooks, R. (2012). The EuroQol group after 25 years. New York: Springer.
Konig, H. H., Ulshofer, A., Gregor, M., von Tirpitz, C., Reinshagen, M., Adler, G., et al. (2002). Validation of the EuroQol questionnaire in patients with inflammatory bowel disease. European Journal of Gastroenterology & Hepatology, 14(11), 1205–1215.
Stark, R. G., Reitmeir, P., Leidl, R., & Konig, H. H. (2010). Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflammatory Bowel Disease, 16(1), 42–51.
Herdman, M., Gudex, C., Lloyd, A., Janssen, M., Kind, P., Parkin, D., et al. (2011). Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research, 20(10), 1727–1736.
Janssen, M. F., Pickard, A. S., Golicki, D., Gudex, C., Niewada, M., Scalone, L., et al. (2013). Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: A multi-country study. Quality of Life Research, 22(7), 1717–1727.
Buchholz, I., Janssen, M. F., Kohlmann, T., & Feng, Y. S. (2018). A systematic review of studies comparing the measurement properties of the three-level and five-level versions of the EQ-5D. Pharmacoeconomics, 36(6), 645–661.
Norton, C., Dibley, L. B., Hart, A., Duncan, J., Emmanuel, A., Knowles, C. H., et al. (2015). Faecal incontinence intervention study (FINS): Self-management booklet information with or without nurse support to improve continence in people with inflammatory bowel disease: Study protocol for a randomized controlled trial. Trials, 16, 444.
Tew, G. A., Carpenter, R., Seed, M., Anderson, S., Langmead, L., Fairhurst, C., et al. (2017). Feasibility of high-intensity interval training and moderate-intensity continuous training in adults with inactive or mildly active Crohn’s disease: Study protocol for a randomised controlled trial. Pilot and Feasibility Studies, 3, 17.
Pentek, M., Lakatos, P. L., Oorsprong, T., Gulacsi, L., Pavlova, M., Groot, W., et al. (2017). Access to biologicals in Crohn’s disease in ten European countries. World Journal of Gastroenterology, 23(34), 6294–6305.
EuroQol, G. (1990). EuroQol–a new facility for the measurement of health-related quality of life. Health Policy, 16(3), 199–208.
Brooks, R. (1996). EuroQol: The current state of play. Health Policy, 37(1), 53–72.
Poor, A. K., Rencz, F., Brodszky, V., Gulacsi, L., Beretzky, Z., Hidvegi, B., et al. (2017). Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L in psoriasis patients. Quality of Life Research, 26(12), 3409–3419.
Janssen, M. F., Birnie, E., Haagsma, J. A., & Bonsel, G. J. (2008). Comparing the standard EQ-5D three-level system with a five-level version. Value in Health, 11(2), 275–284.
van Hout, B., Janssen, M. F., Feng, Y. S., Kohlmann, T., Busschbach, J., Golicki, D., et al. (2012). Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value in Health, 15(5), 708–715.
Dolan, P. (1997). Modeling valuations for EuroQol health states. Medical Care, 35(11), 1095–1108.
Devlin, N. J., Shah, K. K., Feng, Y., Mulhern, B., & van Hout, B. (2018). Valuing health-related quality of life: An EQ-5D-5L value set for England. Health Economics, 27(1), 7–22.
Winship, D. H., Summers, R. W., Singleton, J. W., Best, W. R., Becktel, J. M., Lenk, L. F., et al. (1979). National Cooperative Crohn’s Disease Study: Study design and conduct of the study. Gastroenterology, 77(4 Pt 2), 829–842.
Sandborn, W. J., Feagan, B. G., Hanauer, S. B., Lochs, H., Lofberg, R., Modigliani, R., et al. (2002). A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology, 122(2), 512–530.
Irvine, E. J. (1995). Usual therapy improves perianal Crohn’s disease as measured by a new disease activity index. McMaster IBD Study Group. Journal of Clinical Gastroenterology, 20(1), 27–32.
Losco, A., Vigano, C., Conte, D., Cesana, B. M., & Basilisco, G. (2009). Assessing the activity of perianal Crohn’s disease: Comparison of clinical indices and computer-assisted anal ultrasound. Inflammatory Bowel Disease, 15(5), 742–749.
Ripamonti, C. I. (2012). Pain management. Annals of Oncology, 23(Suppl 10), 294–301.
Janssen, M. F., Birnie, E., & Bonsel, G. J. (2008). Quantification of the level descriptors for the standard EQ-5D three-level system and a five-level version according to two methods. Quality of Life Research, 17(3), 463–473.
Pickard, A. S., De Leon, M. C., Kohlmann, T., Cella, D., & Rosenbloom, S. (2007). Psychometric comparison of the standard EQ-5D to a 5 level version in cancer patients. Medical Care, 45(3), 259–263.
Buchholz, I., Thielker, K., Feng, Y. S., Kupatz, P., & Kohlmann, T. (2015). Measuring changes in health over time using the EQ-5D 3L and 5L: A head-to-head comparison of measurement properties and sensitivity to change in a German inpatient rehabilitation sample. Quality of Life Research, 24(4), 829–835.
Shrout, P. E., & Fleiss, J. L. (1979). Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin, 86(2), 420–428.
Cicchetti, D. (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment, 6(4), 284–290.
Bland, J. M., & Altman, D. G. (1986). Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet, 1(8476), 307–310.
Shannon, C. E. (1948). The mathematical theory of communication. The Bell System Technical Journal, 27, 379–423.
Shannon, C. E., & Weaver, W. (1949). The mathematical theory of communication. Urbana: University of Illinois Press, pp. 104–107.
Swinscow, T., & Campbell, M. (2002). Statistics at square one. London: BMJ.
Ferreira, L. N., Ferreira, P. L., Ribeiro, F. P., & Pereira, L. N. (2016). Comparing the performance of the EQ-5D-3L and the EQ-5D-5L in young Portuguese adults. Health and Quality of Life Outcomes, 14(1), 89.
Yfantopoulos, J., Chantzaras, A., & Kontodimas, S. (2017). Assessment of the psychometric properties of the EQ-5D-3L and EQ-5D-5L instruments in psoriasis. Archives of Dermatological Research, 309(5), 357–370.
Scalone, L., Ciampichini, R., Fagiuoli, S., Gardini, I., Fusco, F., Gaeta, L., et al. (2013). Comparing the performance of the standard EQ-5D 3L with the new version EQ-5D 5L in patients with chronic hepatic diseases. Quality of Life Research, 22(7), 1707–1716.
Jia, Y. X., Cui, F. Q., Li, L., Zhang, D. L., Zhang, G. M., Wang, F. Z., et al. (2014). Comparison between the EQ-5D-5L and the EQ-5D-3L in patients with hepatitis B. Quality of Life Research, 23(8), 2355–2363.
Pan, C. W., Sun, H. P., Wang, X., Ma, Q., Xu, Y., Luo, N., et al. (2015). The EQ-5D-5L index score is more discriminative than the EQ-5D-3L index score in diabetes patients. Quality of Life Research, 24(7), 1767–1774.
Kim, S. H., Kim, H. J., Lee, S. I., & Jo, M. W. (2012). Comparing the psychometric properties of the EQ-5D-3L and EQ-5D-5L in cancer patients in Korea. Quality of Life Research, 21(6), 1065–1073.
Golicki, D., Niewada, M., Karlinska, A., Buczek, J., Kobayashi, A., Janssen, M. F., et al. (2015). Comparing responsiveness of the EQ-5D-5L, EQ-5D-3L and EQ VAS in stroke patients. Quality of Life Research, 24(6), 1555–1563.
Greene, M. E., Rader, K. A., Garellick, G., Malchau, H., Freiberg, A. A., & Rolfson, O. (2015). The EQ-5D-5L improves on the EQ-5D-3L for health-related quality-of-life assessment in patients undergoing total hip arthroplasty. Clinical Orthopaedics and Related Research, 473(11), 3383–3390.
Buxton, M. J., Lacey, L. A., Feagan, B. G., Niecko, T., Miller, D. W., & Townsend, R. J. (2007). Mapping from disease-specific measures to utility: An analysis of the relationships between the Inflammatory Bowel Disease Questionnaire and Crohn’s Disease Activity Index in Crohn’s disease and measures of utility. Value in Health, 10(3), 214–220.
de Dombal, F. T., & Softley, A. (1987). IOIBD report no 1: Observer variation in calculating indices of severity and activity in Crohn’s disease. International Organisation for the Study of Inflammatory Bowel Disease. Gut, 28(4), 474–481.
Khanna, R., Nelson, S. A., Feagan, B. G., D’Haens, G., Sandborn, W. J., Zou, G. Y., et al. (2016). Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database of Systematic Reviews, 8, CD010642.
Peyrin-Biroulet, L., Panes, J., Sandborn, W. J., Vermeire, S., Danese, S., Feagan, B. G., et al. (2016). Defining disease severity in inflammatory bowel diseases: Current and future directions. Clinical Gastroenterology and Hepatology, 14(3), 348–354 e317.
Regueiro, M., Kip, K. E., Schraut, W., Baidoo, L., Sepulveda, A. R., Pesci, M., et al. (2011). Crohn’s disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflammatory Bowel Disease, 17(1), 118–126.
Mulhern, B., Feng, Y., Shah, K., Janssen, M. F., Herdman, M., van Hout, B., et al. (2018). Comparing the UK EQ-5D-3L and english EQ-5D-5L value sets. Pharmacoeconomics, 36(6), 699–713.
Devlin, N., Brazier, J., Pickard, A. S., & Stolk, E. (2018). 3L, 5L, What the L? A NICE Conundrum. Pharmacoeconomics, 36(6), 637–640.
Hernandez Alava, M., Wailoo, A., Grimm, S., Pudney, S., Gomes, M., Sadique, Z., et al. (2018). EQ-5D-5L versus EQ-5D-3L: The Impact on Cost Effectiveness in the United Kingdom. Value in Health, 21(1), 49–56.
Janssen, M. F., Bonsel, G. J., & Luo, N. (2018). Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. Pharmacoeconomics, 36(6), 675–697.
Acknowledgements
This research was supported by the Higher Education Institutional Excellence Program of the Ministry of Human Capacities in the framework of the ‘Financial and Public Services’ research project (1783-3/2018/FEKUTSTRAT) at Corvinus University of Budapest. Fanni Rencz is a postdoctoral research fellow at the Hungarian Academy of Sciences (MTA-BCE PPD 462025). Tamás Cserni’s work was supported by the New National Excellence Programme of the Ministry of Human Capacities of Hungary (ÚNKP-17-2-II-BCE-38). The authors are grateful to Drs Lóránt Gönczi, Eszter Schäfer, Tamás Szamosi, Ferenc Zsigmond and Marianna Rutka for their contribution to the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the National Scientific and Ethical Committee of Hungary (Reference No. 49548-4/2016/EKU).
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Rencz, F., Lakatos, P.L., Gulácsi, L. et al. Validity of the EQ-5D-5L and EQ-5D-3L in patients with Crohn’s disease. Qual Life Res 28, 141–152 (2019). https://doi.org/10.1007/s11136-018-2003-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-018-2003-4