Abstract
Purpose
To compare the quality-adjusted survival of nivolumab plus ipilimumab combination and nivolumab alone versus ipilimumab alone among treatment-naive patients with advanced melanoma based on a minimum 36-month follow-up from the CheckMate 067 trial.
Methods
Overall survival was partitioned into time without symptoms of progression or toxicity (TWiST), time with treatment-related grade ≥ 3 adverse events after randomization but before progression (TOX), and time from progression until end of follow-up or death (REL). Mean quality-adjusted TWiST (Q-TWiST) was calculated by multiplying the mean time spent in each health state by a utility of 1.0 for TWiST and 0.5 for TOX and REL. Sensitivity analyses included varying utilities of TOX and REL; Q-TWiST gains at different follow-up times were calculated using EQ-5D-3L utilities from the trial. Relative Q-TWiST gain of ≥ 10% was considered clinically important.
Results
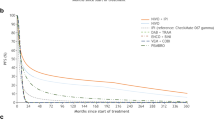
Compared with ipilimumab-treated patients, those who received nivolumab + ipilimumab combination had significantly longer TWiST and TOX but shorter REL; nivolumab-treated patients had significantly longer TWiST, shorter REL, and shorter but statistically nonsignificant TOX. Mean Q-TWiST was highest for nivolumab + ipilimumab (23.5 months; 95% CI 21.9–25.2), followed by nivolumab (21.8 months; 95% CI 20.2–23.4) and ipilimumab (15.3 months; 95% CI 13.9–16.6). Relative Q-TWiST gains were favorable and clinically important for nivolumab + ipilimumab combination (+ 36.81%) and nivolumab alone (+ 29.18%) versus ipilimumab alone. Relative gains increased with follow-up from 3 to 40 months for all comparisons. These gains remained consistent in magnitude and direction in the different sensitivity analyses.
Conclusions
Nivolumab + ipilimumab combination and nivolumab alone resulted in a statistically significant and clinically important improvement in quality-adjusted survival compared with ipilimumab alone.



Similar content being viewed by others
Abbreviations
- M stage:
-
Metastases stage
- PD-1:
-
Programmed death 1
- PD-L1:
-
Programmed death ligand 1
- Q-TWiST:
-
Quality-adjusted time without symptoms or toxicity
- RECIST:
-
Response evaluation criteria in solid tumors
- REL:
-
Time from progression until end of follow-up or death
- TWiST:
-
Time without disease progression or symptoms of toxicity
- TOX:
-
Time with grade ≥ 3 treatment-related adverse events after randomization but before progression
- U:
-
Utilities
- ULN:
-
Upper limit of normal
References
Siegel, R. L., Miller, K. D., & Jemal, A. (2017). Cancer statistics. CA: A Cancer Journal for Clinicians, 67, 7–30.
American Cancer Society. (2018). Cancer facts & figs. 2018. Atlanta: American Cancer Society.
Hodi, F. S., O’Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine, 363(8), 711–723. https://doi.org/10.1056/NEJMoa1003466.
Robert, C., Thomas, L., Bondarenko, I., O’Day, S., Weber, J., Garbe, C., et al. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New England Journal of Medicine, 364(26), 2517–2526. https://doi.org/10.1056/NEJMoa1104621.
Buchbinder, E. I., & Desai, A. (2016). CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. American Journal of Clinical Oncology, 39(1), 98–106. https://doi.org/10.1097/COC.0000000000000239.
Schachter, J., Ribas, A., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2017). Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet, 390(10105), 1853–1862. https://doi.org/10.1016/S0140-6736(17)31601-X.
Long, G. V., Atkinson, V., Ascierto, P. A., Robert, C., Hassel, J. C., Rutkowski, P., et al. (2016). Effect of nivolumab on health-related quality of life in patients with treatment-naive advanced melanoma: Results from the phase III CheckMate 066 study. Annals of Oncology, 27(10), 1940–1946. https://doi.org/10.1093/annonc/mdw265.
Robert, C., Long, G. V., Brady, B., Dutriaux, C., Maio, M., Mortier, L., et al. (2015). Nivolumab in previously untreated melanoma without BRAF mutation. New England Journal of Medicine, 372(4), 320–330. https://doi.org/10.1056/NEJMoa1412082.
Wolchok, J. D., Chiarion-Sileni, V., Gonzalez, R., Rutkowski, P., Grob, J. J., Cowey, C. L., et al. (2017). Overall survival with combined nivolumab and ipilimumab in advanced melanoma. New England Journal of Medicine, 377(14), 1345–1356. https://doi.org/10.1056/NEJMoa1709684.
Schadendorf, D., Larkin, J., Wolchok, J., Hodi, F. S., Chiarion-Sileni, V., Gonzalez, R., et al. (2017). Health-related quality of life results from the phase III CheckMate 067 study. European Journal of Cancer, 82, 80–91. https://doi.org/10.1016/j.ejca.2017.05.031.
European Medicines Agency. (2014). Revised framework for interaction between the European Medicines Agency and patients and consumers and their organisations. http://attwww.ema.europa.eu/docs/en_GB/document_library/Other/2009/12/WC500018013.pdf.
Postmus, D., Richard, S., Bere, N., van Valkenhoef, G., Galinsky, J., Low, E., et al. (2018). Individual trade-offs between possible benefits and risks of cancer treatments: Results from a stated preference study with patients with multiple myeloma. The Oncologist, 23(1), 44–51. https://doi.org/10.1634/theoncologist.2017-0257.
Postmus, D., Mavris, M., Hillege, H. L., Salmonson, T., Ryll, B., Plate, A., et al. (2016). Incorporating patient preferences into drug development and regulatory decision making: Results from a quantitative pilot study with cancer patients, carers, and regulators. Clinical Pharmacology and Therapeutics, 99(5), 548–554. https://doi.org/10.1002/cpt.332.
U. S. Food and Drug Administration. (2017). Plan for issuance of patient-focused drug development guidance under 21st century cures act title III section 3002. https://www.fda.gov/downloads/forindustry/userfees/prescriptiondruguserfee/ucm563618.pdf.
Cherny, N. I., Sullivan, R., Dafni, U., Kerst, J. M., Sobrero, A., Zielinski, C., de Vries, E. G., et al. (2015). A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Annals of Oncology, 26(8), 1547–1573.
Schnipper, L. E., Davidson, N. E., Wollins, D. S., Tyne, C., Blayney, D. W., Blum, D., et al. (2015). American Society of Clinical Oncology Statement: A conceptual framework to assess the value of cancer treatment options. Journal of Clinical Oncology, 33(23), 2563–2577. https://doi.org/10.1200/JCO.2015.61.6706.
Solem, C. T., Kwon, Y., Shah, R. M., Aly, A., & Botteman, M. F. (2018). Systematic review and benchmarking of quality-adjusted time without symptoms or toxicity (Q-TWiST) in oncology. Expert Review of Pharmacoeconomics Outcomes Research, 5, 1–9. https://doi.org/10.1080/14737167.2018.1434414.
Goldhirsch, A., Gelber, R. D., Simes, R. J., Glasziou, P., & Coates, A. S. (1989). Costs and benefits of adjuvant therapy in breast cancer: A quality-adjusted survival analysis. Journal of Clinical Oncology, 7(1), 36–44. https://doi.org/10.1200/JCO.1989.7.1.36.
Revicki, D. A., Feeny, D., Hunt, T. L., & Cole, B. F. (2006). Analyzing oncology clinical trial data using the Q-TWiST method: Clinical importance and sources for health state preference data. Quality of Life Research, 15(3), 411–423. https://doi.org/10.1007/s11136-005-1579-7.
Chung, H. C., Bang, Y., Van Cutsem, E., Kang, Y., Hamamoto, Y., Moiseyenko, V., et al. (2010). (Q)-TWiST analysis of trastuzumab plus fluoropyrimidine/cisplatin(T-XP/FP) versus XP/FP alone as first-line therapy for advanced HER2-positive gastric cancer. Journal of Clinical Oncology, 28, 4048.
Sherrill, B., Wang, J., Kotapati, S., & Chin, K. (2013). Q-TWiST analysis comparing ipilimumab/dacarbazine vs placebo/dacarbazine for patients with stage III/IV melanoma. British Journal of Cancer, 109(1), 8–13. https://doi.org/10.1038/bjc.2013.298.
Kilbridge, K. L., Cole, B. F., Kirkwood, J. M., Haluska, F. G., Atkins, M. A., Ruckdeschel, J. C., et al. (2002). Quality-of-life-adjusted survival analysis of high-dose adjuvant interferon alpha-2b for high-risk melanoma patients using intergroup clinical trial data. Journal of Clinical Oncology, 20(5), 1311–1318. https://doi.org/10.1200/JCO.2002.20.5.1311.
Cole, B. F., Solal-Celigny, P., Gelber, R. D., Lepage, E., Gisselbrecht, C., Reyes, F., et al. (1998). Quality-of-life-adjusted survival analysis of interferon alfa-2b treatment for advanced follicular lymphoma: An aid to clinical decision making. Journal of Clinical Oncology, 16(7), 2339–2344. https://doi.org/10.1200/JCO.1998.16.7.2339.
Patil, S., Figlin, R. A., Hutson, T. E., Michaelson, M. D., Negrier, S., Kim, S. T., et al. (2012). Q-TWiST analysis to estimate overall benefit for patients with metastatic renal cell carcinoma treated in a phase III trial of sunitinib vs interferon-alpha. British Journal of Cancer, 106(10), 1587–1590. https://doi.org/10.1038/bjc.2012.149.
Sloan, J. A., Sargent, D. J., Lindman, J., Allmer, C., Vargas-Chanes, D., Creagan, E. T., et al. (2002). A new graphic for quality adjusted life years (Q-TWiST) survival analysis: The Q-TWiST plot. Quality of Life Research, 11(1), 37–45.
Zbrozek, A. S., Hudes, G., Levy, D., Strahs, A., Berkenblit, A., DeMarinis, R., et al. (2010). Q-TWiST analysis of patients receiving temsirolimus or interferon alpha for treatment of advanced renal cell carcinoma. Pharmacoeconomics, 28(7), 577–584. https://doi.org/10.2165/11535290-000000000-00000.
Zee, B., Cole, B., Li, T., Browman, G., James, K., Johnston, D., et al. (1998). Quality-adjusted time without symptoms or toxicity analysis of interferon maintenance in multiple myeloma. Journal of Clinical Oncology, 16(8), 2834–2839. https://doi.org/10.1200/JCO.1998.16.8.2834.
Lafuma, A., Dreno, B., Delaunay, M., Emery, C., Fagnani, F., Hieke, K., et al. (2001). Economic analysis of adjuvant therapy with interferon alpha-2a in stage II malignant melanoma. European Journal of Cancer, 37(3), 369–375.
Cole, B. F., Gelber, R. D., Kirkwood, J. M., Goldhirsch, A., Barylak, E., & Borden, E. (1996). Quality-of-life-adjusted survival analysis of interferon alfa-2b adjuvant treatment of high-risk resected cutaneous melanoma: An Eastern Cooperative Oncology Group study. Journal of Clinical Oncology, 14(10), 2666–2673. https://doi.org/10.1200/JCO.1996.14.10.2666.
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Cowey, C. L., Lao, C. D., et al. (2015). Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New England Journal of Medicine, 373(1), 23–34. https://doi.org/10.1056/NEJMoa1504030.
Gelber, R. D., Cole, B., Gelber, S., & Goldhirsch, A. (1995). Comparing treatments using quality-adjusted survival: The Q-TWiST method. The American Statistician, 49, 161–169.
Shaw, J. W., Johnson, J. A., & Coons, S. J. (2005). US valuation of the EQ-5D health states: Development and testing of the D1 valuation model. Medical Care, 43(3), 203–220.
Haanen, J., Carbonnel, F., Robert, C., Kerr, K. M., Peters, S., Larkin, J., et al. (2017). Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 28(suppl_4), iv119–iv142. https://doi.org/10.1093/annonc/mdx225.
Gelber, R. D., & Goldhirsch, A. (1986). A new endpoint for the assessment of adjuvant therapy in postmenopausal women with operable breast cancer. Journal of Clinical Oncology, 4(12), 1772–1779. https://doi.org/10.1200/JCO.1986.4.12.1772.
Fenton, J. J., Duberstein, P. R., Kravitz, R. L., Xing, G., Tancredi, D. J., Fiscella, K., et al. (2018). Impact of prognostic discussions on the patient-physician relationship: Prospective cohort study. Journal of Clinical Oncology, 36, 225–230. https://doi.org/10.1200/JCO.2017.75.6288.
Wolchok, J. D., Hoos, A., O’Day, S., Weber, J. S., Hamid, O., Lebbé, C., et al. (2009). Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clinical Cancer Research, 15, 7412–7420.
Jessy, T. (2011). Immunity over inability: The spontaneous regression of cancer. Journal of Natural Science, Biology, and Medicine, 2(1), 43–49. https://doi.org/10.4103/0976-9668.82318.
Seymour, L., Bogaerts, J., Perrone, A., Ford, R., Schwartz, L. H., Mandrekar, S., et al. (2017). iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncology, 18(3), e143–e152. https://doi.org/10.1016/S1470-2045(17)30074-8.
Acknowledgements
We thank the patients and their families, the clinical study teams, and the investigators who participated in the CheckMate 067 trial. Editorial assistance was provided by Kakoli Parai, PhD, and Cara Hunsberger at StemScientific, an Ashfield Company, funded by Bristol-Myers Squibb.
Funding
This study was supported by Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David F. McDermott served as a consultant or advisor for Bristol-Myers Squibb, Pfizer, Merck, Novartis, Eisai, Exelixis, Array BioPharma, and Genentech; and his institution received research funding from Prometheus Laboratories and Bristol-Myers Squibb. Ruchit Shah, Linlin Luo, and Marc Botteman are employed by Pharmerit International. Marc Botteman also reports stock ownership in Pharmerit International. Pharmerit International has received research funding from Bristol-Myers Squibb to conduct this research. Pharmerit International is a global health economics and outcomes research consulting firm that receives research funding and fees related to consulting and other advisory roles from numerous private organizations from the pharmaceutical, biotech, device, and medical industry. Komal Gupte-Singh and Sumati Rao are employed by Bristol-Myers Squibb and own stock in Bristol-Myers Squibb. Javier Sabater was a Bristol-Myers Squibb employee at the time this work was conducted and owns stock in Bristol-Myers Squibb. Meredith M. Regan served as a consultant or advisor for Merck and Ipsen; received funding for travel, accommodations and expenses from Bristol-Myers Squibb; and her institution received research funding from Veridex, OncoGenex, Pfizer, Ipsen, Novartis, Merck, Ferring, Celgene, AstraZeneca, Pierre Fabre, Bayer, and Bristol-Myers Squibb. Michael Atkins served as a consultant or advisor for Genentech, Pfizer, Novartis, GlaxoSmithKline, C-Cam, X4 Pharma, Amgen, Lilly, Alkermes, Infinity Pharmaceuticals, Genoptix, Bristol-Myers Squibb, Nektar, and Merck.
Ethical approval
All procedures performed in the CheckMate 067 study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the CheckMate 067 study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McDermott, D.F., Shah, R., Gupte-Singh, K. et al. Quality-adjusted survival of nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone among treatment-naive patients with advanced melanoma: a quality-adjusted time without symptoms or toxicity (Q-TWiST) analysis. Qual Life Res 28, 109–119 (2019). https://doi.org/10.1007/s11136-018-1984-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-018-1984-3