Abstract
Purpose
Reference values for patient-reported outcome measures are useful for interpretation of results from clinical trials. The study aims were to collect Norwegian SF-36 reference values and compare with data from 1996 to 2002.
Methods
In 2015, SF-36 was sent by mail to a representative sample of the population (N = 6165). Time trends and associations between background variables and SF-36 scale scores were compared by linear regression models.
Results
The 2015 response rate was 36% (N = 2118) versus 67% (N = 2323) in 1996 and 56% (N = 5241) in 2002. Only 5% of the youngest (18–29 years) and 27% of the oldest (>70 years) responded in 2015. Age and educational level were significantly higher in 2015 relative to 1996/2002 (p < .001). The oldest age group in 2015 reported better scores on five of eight scales (p < 0.01), the exceptions being bodily pain, vitality, and mental health compared to 1996/2002 (NS). Overall, the SF-36 scores were relatively stable across surveys, controlled for background variables. In general, the most pronounced changes in 2015 were better scores on the role limitations emotional scale (7.4 points, p < .001) and lower scores on the bodily pain scale (4.6 points, p < .001) than in the 1996/2002 survey.
Conclusions
The low response rate in 2015 suggests that the results, especially among the youngest, should be interpreted with caution. The high response rate among the oldest indicates good representativity for those >70 years. Despite societal changes in Norway the past two decades, HRQoL has remained relatively stable.
Similar content being viewed by others

Introduction
Patient-reported outcome measures (PROMs) represent patients’ own perceptions on their health and well-being [1]. This is important as evaluations by health care professionals may differ considerably from the patients’ own perceptions [2,3,4]. Therefore, PROMs have been recognized as independent outcomes in clinical studies [1] and in health care research in general [2].
PROMs is an umbrella term that includes different dimensions of a person’s health [5] and covers both unidimensional and multidimensional constructs. The latter includes measures of Health-Related Quality of Life (HRQoL) [2, 5]. One of the most widely used HRQoL measures is the Medical Outcome Study 36-item short form (SF-36) [6]. The SF-36 is a generic PROMS tool, i.e., not specific for any population or disease, and assesses HRQOL by eight different scales covering aspects of mental health, physical health, and social functioning [7]. SF-36 has been used in health policy evaluations, clinical practice and research, health interventions, and general population surveying.
Reference data are essential to evaluate whether an individual or a group score is above or below the average for their gender, age, region, country or adjusted for other relevant characteristics. Therefore, reference values for the SF-36 have been developed and published in many countries [8,9,10,11,12,13]. The first Norwegian reference values for the SF-36 were published in 1996 [14]. In 2002, SF-36 data were also collected from a representative sample of the Norwegian population as part of the Norwegian Level of Living Survey conducted by Statistics Norway [15]. In 2017, one paper was published based on the 2002 survey data, aiming to update the normative data and examining the measurement properties of the Norwegian SF-36 [16]. Since then, to our knowledge, no new reference values have been collected or published for the Norwegian population.
Clinicians and other users might question the validity of comparing the relatively old SF-36 reference values with recent patient data as several demographic and lifestyle changes have occurred in Norway and other Western countries during the last decades [17, 18]. In the same period, the number of expected life years has increased, and overall, the Norwegian population leads healthier lives than before. For instance, the percentage of individuals who never exercised decreased by almost 40% in 2015 compared to 1998 [17]. In addition, the proportion of daily smokers has declined steadily over the past 40 years, with approximately 10% daily smokers in 2015 compared to 33% in 2001 [19]. On the other hand, the prevalence of obesity (BMI ≥ 30) increased from 5% in 1995 to 12% in 2015 [17]. Furthermore, there is currently a higher proportion of immigrants in Norway than when the SF-36 reference values were first obtained [18]. These changes have led to an older and more diverse population and may have introduced a greater difference in health-related behaviors between different socio-economic groups. Additionally, research on the performance and stability of the SF-36 in the general population over time is sparse [20,21,22]. One study assessing the stability of HRQoL scores in Norway using the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30) showed that scores remained relatively stable over an eight-year period [23]. Still, the increased focus on the patient perspective in clinical studies has led to a request for updated SF-36 scores.
Study objectives were to (1) present new reference values for the SF-36 and (2) examine the stability of SF-36 scores over the past 19 years, controlling for gender, age, and education, by comparing data from the 2015, 1996, and 2002 surveys.
Materials and methods
Data collection
The data in this report were obtained from three different surveys counting 9837 randomly drawn respondents who completed surveys in 2015 (n = 2118), 1996 (n = 2323), and 2002 (n = 5396). All subjects received a postal questionnaire including the SF-36, and questions regarding sociodemographic variables. The results from the 1996 and 2002 surveys have been presented in detail previously [14,15,16], and thus their methods and results will be presented only in brief. In the present study, the stability of HRQoL was investigated by determining the ability of the SF-36 subscales to identify similarities and differences across the three surveys [21].
In 2015, a total of 6165 subjects, aged 18–80 years, who were representative of the general Norwegian population with respect to age, gender, and place of residence, was randomly drawn by Bring Dialog. In the 1996 survey, a representative sample of 3500 subjects aged 19–80 years was randomly drawn by the Norwegian Government Computer Center (SDS) from the National Register [14]. In the 2002 survey, a sample of 10,000 subjects ≥15 years was randomly drawn from Statistics Norway’s database of demographics/the Norwegian population (BEBAS) [15, 16].
Material
The SF-36
The Norwegian version of the SF-36 version 1 was used in all three surveys. This questionnaire consists of 36 items, grouped into eight multi-item scales that measure physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), and role limitations due to emotional problems (RE) and mental health (MH) [24]. Item scores were transformed to 0–100 point scales (0 = worst, 100 = best) using the SF-36 algorithm [7]. As per the SF-36 algorithm, single imputation was employed meaning that missing values were replaced with the subjects’ mean score for the completed items on the same scale if more than 50% of the scale’s items were completed [24]. Previous international and Norwegian studies have found SF-36 to be a valid, reliable, and suitable measurement of HRQoL [20, 24,25,26,27].
Sociodemographic variables
Only variables measured in the same manner in all three surveys are included in the analysis. All subjects were asked about their age, gender, and highest completed level of education. The 2015 survey included a question about the subjects’ living situation, i.e., whether they were living alone, with other adults, or with children younger than 15 years. Education was divided into three groups based on the level of education: second level, first stage (elementary and/or primary school); second level, second stage (high school); and third level (university college or university).
Statistical analysis
Normally distributed continuous variables are described using the means and standard deviations, while categorical variables are described as percentages. Chi-squared test was used to assess the associations between categorical variables, and independent samples t test was used to assess the differences between two groups in continuous variables. Differences between the three surveys were assessed using one-way ANOVA. Univariate general linear models (GLMs) were fitted to estimate the expected means of the SF-36 scale scores with 99% confidence intervals (CIs) for the 2015, 1996, and 2002 surveys and for different respondent ages, adjusted for education and gender. When comparing the different surveys, all respondents under 18 years were removed; this step was only relevant for the 2002 survey which included respondents from 15 years of age (n = 155). To assess possible associations between the different SF-36 subscale scores and age, survey year, education and gender, eight multivariable linear regression models were fitted, and the corresponding effect sizes are reported as standardized and unstandardized coefficients. Due to multiple testing, null hypotheses were rejected at significance levels of 1% (p < .01). All tests were two-sided. Floor and ceiling effects were considered present if more than 20% of the sample scored the lowest or highest possible score [28]. In the present study, differences in SF-36 subscale scores of 5 points or more were considered clinically relevant [20, 24, 29]. Statistical analyses were performed using IBM SPSS Statistics for Windows, Versions 22.0 and 24.0 (IBM Corp. Armonk, NY).
Results
Reference values from the 2015 survey
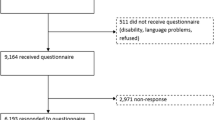
An overview of the inclusion process is presented in Fig. 1. The overall response rate for the 2015 survey was 36%. More females (54%) than males (45%) (p < .001) responded, and the responders (55.7 ± 14.1 years) were significantly older than the non-responders (47.7 ± 15.1 years) (p < .001). The response rates for both men and women were significantly lower in the youngest age groups (<29 years) than in the older age groups (p < .001) (Table 1).
One percent (n = 24) of the questionnaires were returned blank. Missing values on the SF-36 ranged from 0.2% (n = 5, BP items 21 and 22) to 4% (n = 85, GH item 35). Five of the SF-36 scales, PF, RP, BP, SF, and RE had a ceiling effect [28]. Cronbach’s α ranged from .91 (PF) to .79 (MH), indicating an acceptable to excellent internal consistency (Table 2).
The age- and gender-standardized scores for the eight subscales are presented in Table 3. The mean scores decreased with age for all scales except for VT, SF, and MH. Women generally scored slightly lower than men and the gender difference was most pronounced and clinically relevant in the youngest age group (≤29 years), in which women scored 10 points lower than men on the BP and VT scales, and 16 points lower on the RP scale. On the GH subscale, there were only small differences that were not clinically relevant between age groups and gender categories.
Comparisons of SF-36 scores in 2015, 1996 and 2002
The response rate in 2015 was approximately half of those found in 1996 and 2002 (36% vs. 67% and 56%, respectively). Statistically significant differences in sample characteristics were found between the three surveys. Specifically, respondents in the 2015 survey had a significantly higher mean age than those in the 1996 and 2002 surveys (p < .001), and 45% (n = 949) had a university degree in 2015 compared to 28% (n = 643) in 1996 and 25% (n = 1718) in 2002 (p < .001) (Table 4).
To examine the associations between the SF-36 scale scores and sociodemographic factors in the three samples, eight multivariable linear models were estimated (Table 5). To facilitate reading, the number of age groups was limited to four: 18–29, 30–49, 50–64, and 65–96 years. Overall, relatively minor changes were found in the SF-36 scale scores between the three time points: 2015, 1996 and 2002. The participants in the 2015 survey scored statistically significantly (p < .001) higher, indicating better function, on the PF and RE scales than the participants in the 1996 and 2002 surveys. The opposite trend was observed for the GH, BP, and VT scales, i.e., there were statistically significantly (p < .001) lower scores in 2015 than in 1996 and 2002. Clinically relevant differences were detected in BP and RE, in which the 2015 respondents scored approximately five points lower and seven points higher, respectively, than the respondents in 1996 and 2002.
The multivariable regression models (Table 5) showed positive significant associations between high education and all SF-36 scale scores (p < .001) and between high age (65–96 years) and the VT and MH scales. High age was negatively associated with all other SF-36 scale scores (p < .001). Further investigations with sub-analyses indicated that there were some statistically significant effect modifications between survey year and age of respondents (Table 6). The GH decreased by nine points from 1996 to 2015 (p < .01) in the youngest age group, while the VT score decreased by approximately seven points from 2002 to 2015 (p < .01), and these differences were clinically relevant. Participants in the 30- to 49-year-old age group scored statistically significantly lower on GH, BP, and VT in 2015 than in 1996 and 2002 (p < .01), but these differences were only clinically relevant for GH (1996:7.2 points, 2002: 5.8 points) and BP (1996: 6.9 points, 2002: 5.5 points). For the age group 50–64 years, respondents in 2015 scored statistically significantly higher on PF than the respondents in 2002, but the difference was not clinically relevant. The oldest age group in 2015 scored somewhat higher on all scales except for BP, compared to 1996 and 2002, and the differences were statistical significant and clinically relevant for PF, RP, GH, SF, and RE (Table 6).
Discussion
This study provides new Norwegian reference values for the SF-36 based on data from 2118 men and women aged 18–80 years collected in 2015. The randomly drawn sample was representative of the general Norwegian population with respect to age, gender, and place of residence. However, only 36% of the sample responded to the survey. Compared to similar surveys in 1996 and 2002 this response rate was low. However, the stability in scores on all HRQoL domains across the three surveys was high indicating a relatively stable HRQoL in the Norwegian population during the past 19 years, although significant changes were found in certain age groups. Interestingly, the older respondents (≥65 years) in 2015 scored higher on all SF-36 scales than the respondents in 1996 and 2002, except for BP.
The 2015 survey was specifically designed to collect updated reference values for the Norwegian version of SF-36v1 as requested by recent research [16]. However, the low response rate in the 2015 survey questions the representativity of the collected reference values and there are some discrepancies when comparing the sample to the actual composition of the Norwegian population in 2015. About 21% of the population was between 18 and 29 years, while only 5% of this age group participated in the survey. For the older part of population, the opposite pattern was seen. Eighteen percent was 67 years or above, while 27% of the responders were in the same age group [30]. Both findings suggest that the reference values are not fully representative for the Norwegian population with respect to age. Another factor that reduces the representativity is the large proportion of the 2015 sample with a university or college university education. According to Statistics Norway 32% of the population had a higher education in 2015, 41% had finished high school, and 27% had only finished elementary school. In the 2015 sample 45% had higher education, 37% had finished high school, and 18% had finished the lowest education level [31]. These findings bear out two important points. Firstly, the reference values from 2015 should be used with care when performing comparisons for the younger population and for subjects with low education. Secondly, the reference values from 2015 can be assumed to describe the HRQoL in older people better than the 1996 sample since that study had a relatively low response rate for the older parts of the population [14]. The decline in response rate from 67% in 1996 to 36% in 2015 is in accordance with both national [32] and international [33,34,35] findings regarding response rates to postal surveys in the past 15 years. Multiple factors may affect response rates such as the length of the survey, use of pre-notifications, follow-up contact, and survey mode [36]. The same method was used in the three surveys: distribution by mail. Given the digital era of today, one may wonder if the response rate would have been better with an electronic survey. However, some studies have concluded that the use of electronic surveys has a comparable [37] or even lower response rate compared to other survey modes [38]. Suggested explanations have been lack of internet access or computer experience. In 2015, 97% of all households (with at least one person aged <75 years) in Norway had access to the internet [39]. Thus, lack of internet access would therefore probably not have been a challenge in Norway. Also, a study showed that despite having internet access and experience using it, respondents chose to reply on paper rather that online. This may be caused by a fear of sending sensitive personal data over the internet [40]. SF-36 clearly has questions of a sensitive nature, so it is not given that the use of electronic surveys would have increased the response rate in the present surveys. Thus, the external validity of the 2015 data may have been compromised by a potential non-response bias [34]. However, some studies have suggested that higher response rates would not provide different results [23, 33, 41, 42]. Furthermore, a Norwegian study found that HRQoL measured with EORTC QLQ-C30 was relatively stable in two cross-sectional studies eight years apart, despite the fact that the response rate was 33 percentage points higher in the first study than in the second (68% vs. 35%, respectively) [23]. Other studies have suggested that although the estimates may change when including non-responders, the associations may not significantly differ [43, 44].
The scores for the PF, RP, BP, SF, and RE scales showed an extensive ceiling effect. Studies investigating self-perceived health often struggle with ceiling effects [45], and similar results have been reported in previous studies [14, 20]. The ceiling effects detected in the 2015 survey is comparable to the ones reported in 1996 [14] and in 2002 [16]. The biggest differences are an increase of 8.9 percentage points on the RE scale (1996: 71.3% max score vs. 2015: 80.2% max score) and a reduction of 5.6 percentage points on the GH scale (1996: 10.6% max score vs. 2015: 5.0% max score). A possible explanation of the ceiling effect may be the inclusion of a non-hospitalized sample in which scores on the PF, RP, SF, and RE scales are expected to be high. However, it may also suggest that the subjects with the highest burden of disease do not respond, and that the reference values therefore may be biased since results from the sickest individuals are lacking. Still, in comparison with diseased populations, floor effects are of greater concern since these might camouflage differences of clinical importance.
The high proportion of respondents over 70 years may reflect the increased life expectancy in Norway and that elderly who are fit constitute a larger proportion of this age group [46]. The life expectancy at birth in 1996 for women was 81.0 years, compared to 84.1 years in 2015. An even larger improvement is found for men, from 75.3 in 1996 to 80.3 in 2015 [47]. In 2015, the oldest age group scored higher on all SF-36 scale (except BP) compared to these groups in 1996 and 2002. This result can be explained by several factors. First, there has been an increase in healthy life years in the Norwegian population, and in general, eight out of 10 Norwegians report that they have good to very good health [17]. Second, focusing on health promotion and preventing functional decline in community-dwelling elderly are important goals of the Norwegian government, and thus multiple measures have been implemented to attain this goal [48]. However, the higher scores may also represent a healthy bias in the elderly [14]. The elderly with the lowest HRQoL and perhaps the highest burden of disease probably did not participate.
Even if the older age group in 2015 scored higher than in 1996 and 2002, still all physical subscales were negatively affected by increasing age, which is also consistent with previous national [14] and international results [20, 22, 27]. The reduction in physical function may be related to both increased morbidity and the biological aging process which are known to influence physical function through effects such as decline in maximal aerobic capacity, reduced skeletal muscle performance, and changing body composition [49, 50]. For the VT, SF, and MH subscales the opposite trend was observed. The oldest age groups scored higher than the youngest age group who reported the lowest scores on these scales. Previous studies have found similar results of higher VT [14, 27], SF [27], and MH [14, 27] scores in older respondents. Several studies have reported increasing life satisfaction and subjective well-being in older individuals [51, 52]. Perceived subjective well-being and life satisfaction may remain high despite morbidity and/or advanced age. Over time, individuals suffering from chronic conditions or those of advanced age may grow accustomed to their situation [29], and their subjective life expectations may change. An important mediator of this physiological process is “response shift”, which involves adapting and adjusting one’s internal standards, goals, values, and conceptualizations underlying reports of HRQoL [53, 54].
Our results show that education is an important predictor of HRQoL in the Norwegian population. Even though a significantly larger proportion of the respondents in 2015 had completed university/university college education compared to the corresponding proportion in 1996 and 2002, HRQoL remained relatively stable. One explanation for this result may be the absence of potentially important variables in the regression models such as medical conditions [55], lifestyle behavior [56], income [57], and employment status [58]. In previous studies, these factors have been shown to significantly affect HRQoL and may explain why HRQoL did not increase despite the higher education level in the population in 2015.
An important limitation in the present study is the difference in the sample selection of the three surveys. First, both the 2015 and 1996 surveys were designed to collect normative data for SF-36, whereas the 2002 survey was part of an annual cross-sectional study investigating the living conditions in Norway. Second, the SF-36 data from the 2002 survey were part of a larger survey that included telephone or home interview with the respondents regarding health status, presence of chronic diseases, etc. before they received the postal survey containing SF-36 [15]. The respondents in the 2015 and 1996 surveys had no contact with the researchers or other study personnel. Considering that the 2002 survey respondents had contact with the interviewers in advance, this might have affected the motivation for filling out the questionnaire, thus influencing the response rate [16], most probably increasing it somewhat. Third, the layouts and designs of the questionnaires were slightly different in the three different surveys. Even though, the SF-36 questions were identical, one can never rule out that different layouts may have affected the response rates [16]. Another limitation is that the samples’ basic characteristics are statistically significantly different in terms of age, gender, and education level. These differences between samples may have affected the response rates and thus our results. Further, as previously discussed, some differences between the samples were expected a priory, given the demographic changes in the general Norwegian population during the past 19 years, i.e., longer life expectancy, higher levels of education, and maybe larger difference between the very active and healthy on the one hand and the sedentary on the other.
Our overall finding is that relatively minor changes in HRQoL assessed by the SF-36 appear in the Norwegian population over a period of 19 years. This is consistent with findings from other studies, with both cross-sectional and prospective designs [20, 22]. The most pronounced differences were found in the youngest and oldest age groups. This may be interpreted as a result of certain demographic changes, e.g., a longer life expectancy and better health among the oldest. It may also be attributed to a healthy bias in this group and a response bias in the youngest age group.
Conclusion
From a practical standpoint, the present study provided updated Norwegian reference values on the SF-36 v1, which can be used as an anchor point for comparisons with other samples in research and clinical practice. The low response rate, and thus the questionable representativity in the 2015 survey, suggest that the reference values, especially for the youngest age group, should be used and interpreted with caution. The response rate in the oldest age group was high, and the revised reference values can likely be used for people aged >70 years.
Despite the significant changes in Norwegian society over the past two decades, HRQoL has remained relatively stable, hence societal changes may not have affected HRQoL as much as expected. To increase the response rate in future studies, data collection by electronic surveys should be considered, due to the high internet access in Norway and increasing computer experience in the population.
Change history
21 November 2017
In the original publication of the article, the right number of participants included in the analysis should be 2107 and not 2118 as written in the paper. The flow-chart and corrected SF-36 scores for the 2015 data set for this article should have appeared as follows: Fig. 1 and Table 3. These changes did not influence the results. The authors would like to apologize for any inconvenience caused.
References
Reeve, B. B., Mitchell, S. A., Dueck, A. C., Basch, E., Cella, D., Reilly, C. M., et al. (2014). Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. Journal of the National Cancer Institute, 106(7), dju129. doi:10.1093/jnci/dju129.
US Department of Health, Human Services FDA Center for Drug Evaluation Research, US Department of Health, Human Services FDA Center for Biologics Evaluation Research, & US Department of Health, & Human Services FDA Center for Devices Radiological Health. (2006). Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health and Quality of Life Outcomes, 4, 1–20.
Drewes, C., Sagberg, L. M., Jakola, A. S., Gulati, S., & Solheim, O. (2015). Morbidity after intracranial tumor surgery: Sensitivity and specificity of retrospective review of medical records compared with patient-reported outcomes at 30 days. Journal of Neurosurgery, 123(4), 972–977.
Fromme, E. K., Eilers, K. M., Mori, M., Hsieh, Y.-C., & Beer, T. M. (2004). How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. Journal of Clinical Oncology, 22(17), 3485–3490. doi:10.1200/jco.2004.03.025.
Chen, H., Taichman, D. B., & Doyle, R. L. (2008). Health-related quality of life and patient-reported outcomes in pulmonary arterial hypertension. Proceedings of the American Thoracic Society, 5(5), 623–630.
Wee, C. C., Davis, R. B., & Hamel, M. B. (2008). Comparing the SF-12 and SF-36 health status questionnaires in patients with and without obesity. Health and Quality of Life Outcomes, 6(1), 11. doi:10.1186/1477-7525-6-11.
Ware, J. E. (1996). The SF-36 health survey. In B. Spilker (Ed.), Quality of life and pharmacoeconomics in clinical trials (2nd ed., pp. 337–345). Philadelphia: Lippincott Williams & Wilkins.
Apolone, G., & Mosconi, P. (1998). The Italian SF-36 Health Survey: Translation, validation and norming. Journal of Clinical Epidemiology, 51(11), 1025–1036.
Cruz, L. N., Fleck, M. P. D. A., Oliveira, M. R., Camey, S. A., Hoffmann, J. F., Bagattini, Â. M., et al. (2013). Health-related quality of life in Brazil: Normative data for the SF-36 in a general population sample in the south of the country. Ciência & Saúde Coletiva, 18(7), 1911–1921.
Eng, B., Wee, H. L., Wu, Y., Tai, E.-S., & Gandek, B. (2014). Normative data for the Singapore English and Chinese SF-36 version 2 health survey. Annals of the Academy of Medicine, Singapore, 43, 15–23.
Scott, K. M., Tobias, M. I., Sarfati, D., & Haslett, S. J. (1999). SF-36 health survey reliability, validity and norms for New Zealand. Australian and New Zealand Journal of Public Health, 23(4), 401–406.
Sullivan, M., & Karlsson, J. (1998). The Swedish SF-36 Health Survey III. Evaluation of criterion-based validity: Results from normative population. Journal of Clinical Epidemiology, 51(11), 1105–1113.
Hopman, W. M., Towheed, T., Anastassiades, T., Tenenhouse, A., Poliquin, S., Berger, C., et al. (2000). Canadian normative data for the SF-36 health survey. Canadian Medical Association Journal, 163(3), 265–271.
Loge, J. H., & Kaasa, S. (1998). Short form 36 (SF-36) health survey: Normative data from the general Norwegian population. Scandinavian Journal of Public Health, 26(4), 250–258.
Hougen, H. C., Gløboden, M. A., (2004). Samordnet levekårsundersøkelse 2002- tverrsnittsundersøkelsen. Dokumentasjonsrapport [Norwegian]. In Statistisk Sentralbyrå. Avdeling for personstatistikk/Seksjon for intervjuundersøkelse (Ed.).
Garratt, A., & Stavem, K. (2017). Measurement properties and normative data for the Norwegian SF-36: Results from a general population survey. Health and Quality of Life Outcomes, 15(1), 51–61.
Statistics Norway. (2017). Key figures for health. Retrieved February 28, 2017, from https://www.ssb.no/en/helse/nokkeltall/health–245784.
Statistics Norway. (2017). Key figures for the population. Retrieved February 28, 2017, from https://www.ssb.no/en/befolkning/nokkeltall/population.
Statistics Norway. (2017). Smoking habits, 2016. Retrieved March 20, 2017, from https://www.ssb.no/en/helse/statistikker/royk/aar.
Hopman, W. M., Berger, C., Joseph, L., Towheed, T., VandenKerkhof, E., Anastassiades, T., et al. (2006). The natural progression of health-related quality of life: Results of a five-year prospective study of SF-36 scores in a normative population. Quality of Life Research, 15(3), 527–536.
Obidoa, C. A., Reisine, S. L., & Cherniack, M. (2010). How does the SF-36 perform in healthy populations? A structured review of longitudinal studies. Journal of Social, Behavioral, and Health Sciences, 4(1), 2.
Hopman, W. M., Berger, C., Joseph, L., Zhou, W., Prior, J. C., Towheed, T., et al. (2014). Prospectively measured 10-year changes in health-related quality of life and comparison with cross-sectional estimates in a population-based cohort of adult women and men. Quality of Life Research, 23(10), 2707–2721.
Fosså, S. D., Lothe Hess, S., Dahl, A. A., Hjermstad, M. J., & Veenstra, M. (2007). Stability of health-related quality of life in the Norwegian general population and impact of chronic morbidity in individuals with and without a cancer diagnosis. Acta Oncologica, 46(4), 452–461.
Ware, J. E., Snow, K., Kosinski, M., & Gandek, B. (1993). SF-36 Health Survey Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center Hospitals. Inc.
Ware, J. E., Kosinski, M., & Dewey, J. E. (2000). In how to score version two of the SF-36 ® Health Survey. Lincoln, RI: QualityMetric Incorporated.
Loge, J. H., Kaasa, S., Hjermstad, M. J., & Kvien, T. K. (1998). Translation and performance of the Norwegian SF-36 Health Survey in patients with rheumatoid arthritis. I. Data quality, scaling assumptions, reliability, and construct validity. Journal of Clinical Epidemiology, 51(11), 1069–1076.
Hemingway, H., Stafford, M., Stansfeld, S., Shipley, M., & Marmot, M. (1997). Is the SF-36 a valid measure of change in population health? Results from the Whitehall II study. BMJ, 315(7118), 1273–1279.
Di Carlo, A., Lamassa, M., Baldereschi, M., Pracucci, G., Consoli, D., Wolfe, C. D., et al. (2006). Risk factors and outcome of subtypes of ischemic stroke. Data from a multicenter multinational hospital-based registry. The European Community Stroke Project. Journal of the Neurological Sciences, 244(1), 143–150.
Wang, H.-M., Beyer, M., Gensichen, J., & Gerlach, F. M. (2008). Health-related quality of life among general practice patients with differing chronic diseases in Germany: Cross sectional survey. [journal article]. BMC Public Health, 8(1), 246. doi:10.1186/1471-2458-8-246.
Statistics Norway (2016). Population projections, 2016-2100. Retrieved May 05, 2017, from https://www.ssb.no/en/befolkning/statistikker/folkfram.
Statistics Norway (2017). Key figures on education. Retrieved April 11, 2017, from https://www.ssb.no/en/utdanning/nokkeltall/education.
Abrahamsen, R., Svendsen, M. V., Henneberger, P. K., Gundersen, G. F., Torén, K., Kongerud, J., et al. (2016). Non-response in a cross-sectional study of respiratory health in Norway. British Medical Journal Open, 6(1), e009912.
Christensen, A. I., Ekholm, O., Kristensen, P. L., Larsen, F. B., Vinding, A. L., Glümer, C., et al. (2015). The effect of multiple reminders on response patterns in a Danish health survey. The European Journal of Public Health, 25(1), 156–161.
Mannetje, A. T., Eng, A., Douwes, J., Ellison-Loschmann, L., McLean, D., & Pearce, N. (2011). Determinants of non-response in an occupational exposure and health survey in New Zealand. Australian and New Zealand Journal of Public Health, 35(3), 256–263.
Rönmark, E. P., Ekerljung, L., Lötvall, J., Torén, K., Rönmark, E., & Lundbäck, B. (2009). Large scale questionnaire survey on respiratory health in Sweden: Effects of late-and non-response. Respiratory Medicine, 103(12), 1807–1815.
Sheehan, K. B. (2001). E-mail survey response rates: A review. Journal of Computer-Mediated Communication, 6(2). doi:10.1111/j.1083-6101.2001.tb00117.x. Retrieved Aug 12, 2017.
Kaplowitz, M. D., Hadlock, T. D., & Levine, R. (2004). A comparison of web and mail survey response rates. Public Opinion Quarterly, 68(1), 94–101.
Manfreda, K. L., Bosnjak, M., Berzelak, J., Haas, I., Vehovar, V., & Berzelak, N. (2008). Web surveys versus other survey modes: A meta-analysis comparing response rates. Journal of the Market Research Society, 50(1), 79.
Statistics Norway. (2015). ICT usage in households, 2015, 2nd quarter. https://www.ssb.no/en/teknologi-og-innovasjon/statistikker/ikthus/aar/2015-10-01. Retrieved from 10 April 2017.
Scott, A., Jeon, S.-H., Joyce, C. M., Humphreys, J. S., Kalb, G., Witt, J., et al. (2011). A randomised trial and economic evaluation of the effect of response mode on response rate, response bias, and item non-response in a survey of doctors. BMC Medical Research Methodology, 11(1), 126.
Lee, S., Brown, E. R., Grant, D., Belin, T. R., & Brick, J. M. (2009). Exploring nonresponse bias in a health survey using neighborhood characteristics. American Journal of Public Health, 99(10), 1811–1817.
Groves, R. M., & Peytcheva, E. (2008). The impact of nonresponse rates on nonresponse bias a meta-analysis. Public Opinion Quarterly, 72(2), 167–189.
Brøgger, J., Bakke, P., Eide, G. E., & Gulsvik, A. (2003). Contribution of follow-up of nonresponders to prevalence and risk estimates: A Norwegian respiratory health survey. American Journal of Epidemiology, 157(6), 558–566.
Mealing, N. M., Banks, E., Jorm, L. R., Steel, D. G., Clements, M. S., & Rogers, K. D. (2010). Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Medical Research Methodology, 10(1), 26.
Austin, P. C. (2002). Bayesian extensions of the Tobit model for analyzing measures of health status. Medical Decision Making, 22(2), 152–162.
Syse, A., Pham, D.Q, Keilman N (2016). Befolkningsframskrivinger 2016-2100: Dødelighet og levealder [Norwegian]. Økonomiske analyser (Vol. 3).
Statistics Norway (2016). Deaths, 2015. Retrieved April 9, 2017, from https://www.ssb.no/en/befolkning/statistikker/dode/aar/2016-03-09.
St.meld. nr. 47 (2008-2009). (2009). Samhandlingsreformen - Rett behandling – på rett sted – til rett tid [Norwegian]. Retrieved March 20, 2017, from https://www.regjeringen.no/no/dokumenter/stmeld-nr-47-2008-2009-/id567201/sec1.
Janssen, I., & Ross, R. (2005). Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. Journal of Nutrition Health and Aging, 9(6), 408.
Chodzko-Zajko, W. J., Proctor, D. N., Singh, M. A. F., Minson, C. T., Nigg, C. R., Salem, G. J., et al. (2009). Exercise and physical activity for older adults. Medicine and Science in Sports and Exercise, 41(7), 1510–1530.
Deaton, A. (2007). Income, aging, health and wellbeing around the world: Evidence from the Gallup World Poll. National Bureau of Economic Research.
Frijters, P., & Beatton, T. (2012). The mystery of the U-shaped relationship between happiness and age. Journal of Economic Behavior & Organization, 82(2), 525–542.
Sprangers, M. A., & Schwartz, C. E. (1999). Integrating response shift into health-related quality of life research: A theoretical model. Social Science & Medicine. doi:10.1016/s0277-9536(99)00045-3.
Hansen, T., & Slagsvold, B. (2012). The age and subjective well-being paradox revisited: A multidimensional perspective. Norsk epidemiologi, 22(2), 187–195.
Orfila, F., Ferrer, M., Lamarca, R., Tebe, C., Domingo-Salvany, A., & Alonso, J. (2006). Gender differences in health-related quality of life among the elderly: The role of objective functional capacity and chronic conditions. Social Science and Medicine, 63(9), 2367–2380.
Blanchard, C. M., Stein, K. D., Baker, F., Dent, M. F., Denniston, M. M., Courneya, K. S., et al. (2004). Association between current lifestyle behaviors and health-related quality of life in breast, colorectal, and prostate cancer survivors. Psychology & Health, 19(1), 1–13.
Pappa, E., Kontodimopoulos, N., Papadopoulos, A. A., & Niakas, D. (2009). Assessing the socio-economic and demographic impact on health-related quality of life: Evidence from Greece. International Journal of Public Health, 54(4), 241–249.
Mein, G., Martikainen, P., Hemingway, H., Stansfeld, S., & Marmot, M. (2003). Is retirement good or bad for mental and physical health functioning? Whitehall II longitudinal study of civil servants. Journal of Epidemiology and Community Health, 57(1), 46–49.
Acknowledgements
The authors would like to thank Professor Are Hugo Pripp and PhD Milada Småstuen for their kind help with the statistical analysis and interpretation.
Funding
The 2015 study received funding from Holes legat, the Cancer Trust, St Olav’s Hospital, Trondheim University Hospital (Project No. 35/15) and the Norwegian Hospital Foundation (Project No. 335007).
Author information
Authors and Affiliations
Contributions
Conceptualization: JHL, MJH. Data curation: ELJ, AB, KSG. Formal analysis: ELJ. Funding acquisition: MJH, SDF, JHL, SK. Investigation: KSG, JHL. Methodology: MJH, TM, KSG, JHL. Project administration: MJH, KSG, JHL. Resources: MJH, SDF, JHL, SK. Supervision: MJH, AB, JHL, KSG, TM, SDF. Visualization: ELJ. Writing-original draft: ELJ, AB. Writing-review & editing: MJH, ELJ, AB, JHL, TM, SDF, KSG, NA, SK.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
All surveys included in this study were conducted according to Norwegian regulations for surveys. The Regional Committee for Medical and Health Research Ethics (REC) South East Norway approved the 1996 and 2015 surveys, while The Norwegian Centre for Research Data (NSD) approved the 2002 survey. Return of the questionnaires was regarded as informed consent.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study at the time of the survey.
Additional information
Some of the included data (the 2002 survey) were obtained from « Levekårsundersøkelsen 2002 Tverrsnitt, Tema: Helse » [The Norwegian Level of Living Survey 2002 Cross-sectional, theme: health”]. Anonymized data are available from Statistisk sentralbyrå [Statistics Norway] through Norsk samfunnsvitenskapelig datatjeneste AS (NSD) [Norwegian Center for Research Data]. Neither Statistics Norway nor NSD are responsible for the data analysis or the interpretations in the present study.
The original version of this article was revised due to a retrospective Open Access order.
Rights and permissions
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jacobsen, E.L., Bye, A., Aass, N. et al. Norwegian reference values for the Short-Form Health Survey 36: development over time. Qual Life Res 27, 1201–1212 (2018). https://doi.org/10.1007/s11136-017-1684-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-017-1684-4