Abstract
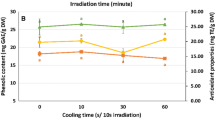
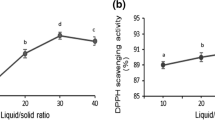
The purpose of this study was to analyze the extraction efficiency of antioxidants from mango peel by comparing two techniques: microwave-assisted (MAE) and traditional solvent (TE) extraction. The number of extraction steps, water content in the extractant, peel weight-to-solvent volume ratio in extractions and extraction time all had an influence on obtaining extracts with high antioxidant capacity, but the extraction technique and the water content in the extractant were the factors with the greatest effect. Using three steps, a water content of 50 % in the ethanol:water extractant, an extraction time of 60 min and a weight-to-volume ratio of 1:10 or 1:50 (w/v) led to the highest antioxidant activity and phytochemicals content in extracts. The extraction time needed to extract phytochemicals from mango peel was similar when MAE and TE were used. However, the antioxidant capacity and phytochemical content were around 1.5–6.0 times higher in the extracts obtained by MAE.

Similar content being viewed by others
Abbreviations
- AAC:
-
Antioxidant activity coefficient
- \( \mathrm{ABT}{{\mathrm{S}}^{{\bullet +}}} \) :
-
2,2′-Azino-bis-(3-ethylbenzothiazoline)-6-sulfonic acid radical
- ANOVA:
-
Analysis of variance
- CCD:
-
Central composite design
- CF:
-
Contribution factor
- \( \mathrm{DPP}{{\mathrm{H}}^{\bullet }} \) :
-
2,2-Diphenyl-1-picrylhydrazyl radical
- DW:
-
Dry matter basis
- EV:
-
Experimental values
- LEs:
-
Leucoanthocyanidin equivalents
- MAE:
-
Microwave-assisted extraction
- MW:
-
Microwave
- PV:
-
Predicted values
- p :
-
Probability
- PVPP:
-
Polyvinylpolypyrrolidone
- SEE:
-
Standard error of the estimates
- TBARS:
-
Thiobarbituric acid reactive substances
- TAEs:
-
Tannic acid equivalents
- TE:
-
Traditional solvent extraction
- TEAC:
-
Trolox equivalent antioxidant capacity
References
European Union (1995) Directive 1995/2/EC of the European Parliament and of the Council of 20 February 1995 on food additives other than colours and sweeteners. Off J Eur Comm L61
Schieber A, Berardini N, Carle R (2003) Identification of flavonol and xanthone glycosides from mango (Mangifera indica L. cv. “Tommy Atkins”) peels by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem 51:5006–5011
Berardini N, Carle R, Schieber A (2004) Characterization of gallotannins and benzophenone derivatives from mango (Mangifera indica L. cv. Tommy Atkins) peels, pulp and kernels by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 18:2208–2216
Berardini N, Fezer R, Conrad J, Beifuss U, Carle R, Schieber A (2005) Screening of mango (Mangifera indica L.) cultivars for their contents of flavonol O- and xanthone C-glycosides, anthocyanins, and pectin. J Agric Food Chem 53:1563–1570
Barreto JC, Trevisan MTS, Hull WE, Erben G, de Brito ES, Pfundstein B, Wurtele G, Spiegelhalder B, Owen RW (2008) Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves and peel of mango (Mangifera indica L.). J Agric Food Chem 56:5599–5610
Ribeiro SMR, Barbosa LCA, Queiroz JH, Knödler M, Schieber A (2008) Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem 110:620–626
Dorta E, Lobo MG, González M (2011) Reutilization of mango by-products: study of the effect of extraction solvent and temperature on their antioxidant properties. J Food Sci 77:C80–C88
González M, González V (2010) Sample preparation of tropical and subtropical fruit biowastes to determine antioxidant phytochemicals. Anal Methods UK 2:1842–1866
Güçlü-Üstündağ Ö, Mazza G (2009) Effects of pressurized low polarity water extraction parameters on antioxidant properties and composition of cow cockle seed extracts. Plant Foods Hum Nutr 64:32–38
Ghafoor K, AL-Juhaimi FY, Choi YH (2012) Supercritical fluid extraction of phenolic compounds and antioxidants from grape (Vitis labrusca B.) seeds. Plant Foods Hum Nutr 67:407–414
Pan Y, Wang K, Huang S, Wang H, Mu X, He C, Ji X, Zhang J, Huang F (2008) Antioxidant activity of microwave assisted extract of longan (Dimocarpus Longan Lour.) peel. Food Chem 106:1264–1270
Desai M, Parikh J, Parikh PA (2010) Extraction of natural products using microwaves as a heat source. Sep Purif Rev 39:1–32
Routray W, Orsat V (2012) Microwave-assisted extraction of flavonoids: a review. Food Bioprocess Technol 5:409–424
Pérez-Serradilla JA, Luque de Castro MD (2011) Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem 124:1652–1659
Dorta E, Lobo MG, González M (2013) Optimization of factors affecting extraction of antioxidants from mango seed. Food Bioprocess Technol 6:1067–1081
Jiang IY, He S, Pan YJ, Sun CR (2010) Bioassay-guided isolation and EPR-assisted antioxidant evaluation of two valuable compounds from mango peels. Food Chem 119:1285–1292
Kim H, Moon JY, Kim H, Lee DS, Cho M, Choi HK, Kim YS, Mosaddik A, Cho SK (2010) Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem 121:429–436
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
González-Montelongo R, Lobo MG, González M (2010) The effect of extraction temperature, time and number of steps on the antioxidant capacity of methanolic banana peel extracts. Sep Purif Technol 71:347–355
Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73:239–244
Miller HE (1971) A simplified method for the evaluation of antioxidants. J Am Oil Chem Soc 48:91
Zhu H, Wang Y, Liu Y, Xia Y, Tang T (2010) Analysis of flavonoids in Portulaca oleracea L. by UV-Vis spectrophotometry with comparative study on different extraction technologies. Food Anal Method 3:90–97
Hemwimon S, Pavasant P, Shotipruk A (2007) Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep Purif Technol 54:44–50
Nepote V, Grosso NR, Guzmán CA (2005) Optimization of extraction of phenolic antioxidants from peanut skins. J Sci Food Agric 85:33–38
Kaufmann B, Christen P (2002) Recent extraction techniques for natural products: microwave-assisted extraction and pressurised solvent extraction. Phytochem Anal 13:105–113
Liazid A, Palma M, Brigui J, Barroso CB (2007) Investigation on phenolic compounds stability during microwave-assisted extraction. J Chromatogr A 1140:29–34
Sun Y, Liao X, Wang Z, Hu X, Chen F (2007) Optimization of microwave-assisted extraction of anthocyanins in red raspberries and identification of anthocyanin of extracts using high-performance liquid chromatography-mass spectrometry. Eur Food Res Technol 225:511–523
Acknowledgments
The Spanish INIA awarded Eva Dorta a PhD INIA grant. Mónica González would like to thank the Spanish National Research Council (CSIC) for a contract in the JAEdoc program, financed with the involvement of the European Social Fund (ESF). This research was supported through the RTA2006-00187 project, also financed by the INIA.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 40 kb)
Rights and permissions
About this article
Cite this article
Dorta, E., Lobo, M.G. & González, M. Improving the Efficiency of Antioxidant Extraction from Mango Peel by Using Microwave-assisted Extraction. Plant Foods Hum Nutr 68, 190–199 (2013). https://doi.org/10.1007/s11130-013-0350-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-013-0350-4