Abstract
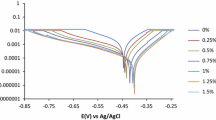
The inhibition effect of Rhodanine on the corrosion performance of mild steel (MS) was studied in 0.5 M HCl solution. For this purpose, potentiodynamic polarization, electrochemical impedance spectroscopy, and long-term corrosion tests including hydrogen evolution and change in open-cirquit potential during immersion time were used. To determine activation energy of corrosion process, potentiodynamic polarization curves were also obtained in a temperature range from 298 to 328 K. The results show that Rhodanine effectively inhibits the MS corrosion in hydrochloric acid solution.
Similar content being viewed by others
REFERENCES
Khaled, K.F. and Hackerman, N., Electrochim. Acta, 2003, vol. 48, p. 2715.
Elkadi, L., Mernari, B., Traisnel, M., Bentiss, F., and Lagrenee, M., Corros. Sci., 2000, vol. 42, p. 703.
Quraishi, M.A., Ansari, F.A., and Jamal, D., Mater. Chem. Phys., 2002, vol. 77, p. 687.
Quraishi, M.A. and Jamal, D., Mater. Chem. Phys., 2003, vol. 78, p. 608.
Ebenso, E.E., Ekpe, U.J., Ita, B.I., Offiong, O.E., and Ibok, U.J., Mater. Chem. Phys., 1999, vol. 60, p. 79.
Kertit, S. and Hammouti, B., Appl. Surf. Sci., 1996, vol. 93, p. 59.
Chetouani, A., Aouniti, A., Hammouti, B., Benchat, N., Benhadda, T., and Kertit, S., Corros. Sci., 2003, vol. 45, p. 1675.
Abd, S.A. and El-Maksoud, Corros. Sci., 2002, vol. 44, p. 803.
Sing, W.T., Lee, C.L., Yeo, S.L., Lim, S.P., and Sim, M.M., Bioorg. Med. Chem. Lett., 2001, vol. 11, p. 91.
El-Dissouky, A., El-Bindary, A.A., El-Sonbati, A.Z., and Hilali, A.S., Spectrochim. Acta A, 2001, vol. 57, p. 1163.
Tang, E., Yang, G., and Yin, J., Spectrochim. Acta A, 2003, vol. 59, p. 651.
Abdallah, M., Corros. Sci., 2002, vol. 44, p. 717.
Quaraishi, M.A. and Rawat, J., Mater. Chem. Phys., 2002, vol. 77, p. 43.
Bentis, F., Lagrenee, M., Traisnel, M., and Hornez, J.C., Corros. Sci., 1999, vol. 41, p. 789.
Muller, B., React. Funct. Polym., 1999, vol. 39, p. 165.
El-Etre, A.Y., Corros. Sci., 2003, vol. 451, p. 2485.
Lagrenee, M., Mernari, B., Bouanis, M., Traisnel, M., and Bentiss, F., Corros. Sci., 2002, vol. 44, p. 573.
Khalad, K.F., Electrochim. Acta, 2003, vol. 48, p. 2493.
Author information
Authors and Affiliations
Additional information
__________
From Zashchita Metallov, Vol. 41, No. 6, 2005, pp. 628–632.
Original English Text Copyright © 2005 by Solmaz, Kardas, Yazici, Erbil.
The text was submitted by authors in English.
Rights and permissions
About this article
Cite this article
Solmaz, R., Kardas, G., Yazici, B. et al. Inhibition Effect of Rhodanine for Corrosion of Mild Steel in Hydrochloric Acid Solution. Prot Met 41, 581–585 (2005). https://doi.org/10.1007/s11124-005-0083-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11124-005-0083-3