Abstract
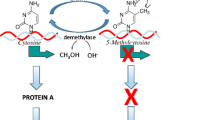
The Strong African American Family (SAAF) program has been shown to have a variety of short and long-term benefits for participating youth and families. However, biological mechanisms potentially influencing long-term effects on resilience in young adulthood have not been examined. In the current investigation, we examine the effects of SAAF on methylation of the OXTR gene in young adulthood, focusing on a regulatory region previously identified to be both responsive to stress and implicated in resilience. Using the subsample of participants from the original study for whom methylation data was available (N = 388), we replicated the previously reported G × E effect on prevention of early substance use and then examined whether there would also be a moderated effect on OXTR methylation in early adulthood, with “s” allele carriers, but not “LL” participants, showing a significant indirect effect of SAAF on OXTR methylation. Results suggest that for susceptible youth (i.e., “s” allele carriers), preventive intervention may “get under the skin,” in a manner potentially beneficial for long-term outcomes. Implications for examination of OXTR methylation in future prevention research are discussed.



Similar content being viewed by others
References
Baumgartner, T., Heinrichs, M., Vonlanthen, A., Fischbacker, U., & Fehr, E. (2008). Oxytocin shapes the neural circuitry of trust and trust adaptations in humans. Neuron, 58, 639–650. doi:10.1016/j.neuron.2008.04.009.
Beach, S. R. H., Brody, G. H., Lei, M. K., Kim, S., Cui, J., & Philibert, R. A. (2014). Is serotonin transporter genotype associated with epigenetic susceptibility or vulnerability? Examination of the impact of socioeconomic status risk on African American youth. Development and Psychopathology, 26, 289–304. doi:10.1017/S0954579413000990.
Beach, S. R. H., Lei, M. K., Brody, G. H., Dogan, M. V., & Philibert, R. A. (2015). Higher levels of protective parenting are associated with better young adult health: exploration of mediation through epigenetic influences on pro-inflammatory processes. Frontiers in Psychology, 6, 676. doi:10.3389/fpsyg.2015.00676.
Beach, S. R. H., Brody, G. H., Barton, A.W., Philibert, R. A. (2016). Exploring genetic moderators and epigenetic mediators of contextual and family effects: From G × E to epigenetics. Development and Psychopathology. doi:10.1017/S0954579416000882.
Belsky, J., & Pluess, M. (2009). Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin, 135, 885–908. doi:10.1037/a0017376.
Bradley, S. L., Dodelzon, K., Sandhu, H. K., & Philibert, R. A. (2005). Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 136B, 58–61. doi:10.1002/ajmg.b.30185.
Brenet, F., Moh, M., Funk, P., Feierstein, E., Viale, A. J., Socci, N. D., & Scandura, J. M. (2011). DNA methylation of the first exon is tightly linked to transcriptional silencing. PLoS ONE, 6, e14524. doi:10.1371/journal.pone.0014524.
Brody, G. H., Murry, V. M., Kogan, S. M., Gerrard, M., Gibbons, F. X., Molgaard, V., & Wills, T. A. (2006). The strong African American families program: a cluster-randomized prevention trial of long-term effects and a mediational model. Journal of Consulting and Clinical Psychology, 74, 356–366. doi:10.1037/0022-006X.74.2.356.
Brody, G. H., Beach, S. R. H., Philibert, R. A., Chen, Y.-F., & Murry, V. M. (2009). Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: Gene × environment hypotheses tested via a randomized prevention design. Child Development, 80, 645–661. doi:10.1111/j.1467-8624.2009.01288.x.
Brook, J. S., & Newcomb, M. D. (1995). Childhood aggression and unconventionality: Impact on later academic achievement, drug use, and workforce involvement. Journal of Genetic Psychology, 156, 393–410. doi:10.1080/00221325.1995.9914832.
Caspi, A., Hariri, A. R., Holmes, A., Uher, R., & Moffit, T. E. (2010). Genetic sensitivity to the environment: The case of the serotonin transporter gene (5-HTT) and its implications for studying complex diseases and traits. American Journal of Psychiatry, 167, 509–527. doi:10.1176/appi.ajp.2010.09101452.
Cecil, C. A. M., Lysenko, L. J., Jaffee, S. R., Pingault, J. B., Smith, R. G., Relton, C. L., & Barker, E. D. (2014). Environmental risk, oxytocin receptor gene (OXTR) methylation and youth callous-unemotional traits: A 13-year longitudinal study. Molecular Psychiatry, 19, 1071–1077. doi:10.1038/mp.2014.95.
Choi, W. S., Gilpin, E. A., Farkas, A. J., & Pierce, J. P. (2001). Determining the probability of future smoking among adolescents. Addiction, 96, 313–323. doi:10.1046/j.1360-0443.2001.96231315.x.
Dadds, M. R., Moul, C., Cauchi, A., Dobson-Stone, C., Hawes, D. J., Brennanm, J., & Ebstein, R. E. (2014). Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Development and Psychopathology, 26, 33–40. doi:10.1017/S09545794130000497.
Dogan, M. V., Shields, B., Cutrona, C., Gao, L., Gibbons, F. X., Simons, R., Monick, M., Brody, G. H., Tan, K., Beach, S. R. H., & Philibert, R. A. (2014). The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women. BMC Genomics, 15, 151. doi:10.1186/1471-2164-15-151.
Falush, D., Stephens, M., & Pritchard, J. K. (2007). Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Molecular Ecology Notes, 155, 945–959. doi:10.1111/j.1471-8286.2007.01758.x.
Galfi, M., Radacs, M., Juhasz, A., Molner, A., & Laszlo, F. A. (2005). Serotonin-induced enhancement of vasopressin and oxytocin secretion in neurohypophyseal tissue culture. Regulatory Peptides, 127, 225–231. doi:10.1016/j.regpep.2004.12.009.
Gamer, M., Zurowski, B., & Burchel, C. (2010). Different amygdala subregions mediate valence related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Science, 107, 9400–9405. doi:10.1073/pnas.1000985107.
Halder, I., Yang, B.-Z., Kranzler, H. R., Stein, M. B., Shriver, M. D., & Gelernter, J. (2009). Measurement of admixture proportions and description of admixture structure in different U.S. populations. Human Mutation, 30, 1299–1309. doi:10.1002/humu.21045.
Houseman, E. A., Accomando, W. P., Koestler, D. C., Christensen, B. C., Marsit, C. J., Nelson, H. H., Wiencke, J. K., & Kelsey, K. T. (2012). DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics, 13, 86–101. doi:10.1186/1471-2105-13-86.
Kaufman, J., Ying, B., Douglas-Palumberi, H., Cruse-Artus, M., Lipshitz, D., Krystal, J. H., & Gelernter, J. (2007). Genetic and environmental predictors of early alcohol use. Biological Psychiatry, 61, 1228–1234. doi:10.1086/j.biopsych.2006.06.039.
Kim, Y.-R., Kim, J.-H., Kim, M. J., & Treasure, J. (2014). Differential methylation of the oxytocin receptor gene in patients with anorexia nervosa: A pilot study. PLoS ONE, 9, 1–4. doi:10.1371/journal.pone.0088673.
Kogan, S. M., Brody, G. H., Beach, S. R. H., & Philibert, R. A. (2010). 5-HTTLPR status moderates the effect of early adolescent substance use on risky sexual behavior. Health Psychology, 29, 471–476. doi:10.1037/a0020594.
Kosfeld, M., Heinrichs, M., Zak, P. J., Fischbacker, U., & Fehr, E. (2005). Oxytocin increases trust in humans. Nature, 435, 673–676. doi:10.1038/nature03701.
Kovacs, G. L., Zoltan, S., & Szabo, G. (1998). Oxytocin and addiction: A review. Psychoneuroendocrinology, 23, 945–962. doi:10.1016/s0306-4530(98)00064-x.
Kumsta, R., Hummel, E., Chen, F. S., & Heinrichs, M. (2013). Epigenetic regulation of the oxytocin receptor gene: Implications fr behavioral neuroscience. Frontiers in Neuroscience, 7, 1–6. doi:10.3389/fnins.2013.00083.
Kusui, C., Kimura, T., Ogita, K., Nakamura, H., Matsumara, Y., Koyama, M., Azuma, C., & Murata, Y. (2001). DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochemical and Biophysical Research Communications, 289, 681–686. doi:10.1006/bbrc.2001.6024.
Landecker, H., & Panofsky, A. (2013). From social structure to gene regulation, and back: A critical introduction to environmental epigenetics for sociology. Annual Review of Sociology, 39, 333–357. doi:10.1146/annureview-soc.071312-145707.
MacDonald, K., & MacDonald, T. M. (2010). The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry, 18, 1–21. doi:10.3109/10673220903523615.
MacPhee, D., Lunkenheimer, E., & Riggs, N. R. (2015). Resilience as regulation of developmental and family processes. Family Relations, 64, 153–175. doi:10.1111/fare.12100.
McGregor, J. S., & Bowen, M. T. (2012). Breaking the loop: Oxytocin as a potential treatment for drug addiction. Hormones and Behavior, 61, 331–339. doi:10.1016/j.yhbeh.2011.12.001.
Meloni, M. (2014). The social brain meets the reactive genome: Neuroscience, epigenetics and the new social biology. Frontiers in Human Neuroscience, 8, 1–12. doi:10.3389/fnhum.2014.00309.
Mottolese, R., Redoute, J., Costes, N., Le Bars, D., & Sirigu, A. (2014). Switching brain serotonin with oxytocin. Proceedings of the National Academy of Science, 111, 8637–8642. doi:10.1073/pnas.1319810111.
Nakamura, M., Ueno, S., Sano, A., & Tanabe, H. (2000). The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Molecular Psychiatry, 5, 32–38. doi:10.1038/sj.mp.4000698.
Olff, M., Frijing, J. L., Kubzansky, L. D., Bradley, B., Ellenbogen, M. A., Cardoso, C., Bartz, J. A., Yee, J. R., & van Zuiden, M. (2013). The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology, 38, 1883–1894. doi:10.1016/j.psyneuen.2013.06.019.
Philibert, R. A., Sandhu, H., Hollenbeck, N., Gunter, T., Adams, W., & Madan, A. (2008). The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 147B, 543–9. doi:10.1002/ajmg.b.30657.
Pidsley, R., Wong, C. C. Y., Volta, M., Lunnon, K., Mill, J., & Schalkwyk, L. C. (2013). A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genomics, 14, 293–302. doi:10.1186/1471-2164-14-293.
Preacher, K. J., Rucker, D. D., & Hayes, A. F. (2007). Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research, 42, 185–227. doi:10.1080/00273170701341316.
Proctor, B. D., & Dalaker, J. (2003). Poverty in the United States: 2002. U.S. Census Bureau, Current Population Reports, P60-222, U.S. Government Printing Office, Washington, DC.
Puglia, M. H., Lillard, T. S., Morris, J. P., & Connelly, J. J. (2015). Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proceedings of the National Academy of Science, 112, 3308–3313. doi:10.1073/pnas.1422096112.
Raleigh, M. J., McGuire, M. T., Brammer, G. L., & Yuwiler, A. (1984). Social and environmental influences on blood serotonin concentrations in monkeys. Archives of General Psychiatry, 41, 405–410. doi:10.1001/archpsyc.1984.01790150095013.
Reinius, L. E., Acevedo, N., Joerink, M., Pershagen, G., Dahlén, S. E., Greco, D., Söderhäll, C., Scheynius, A., & Kere, J. (2012). Differential DNA methylation in purified human blood cells: Implications for cell lineage and studies on disease susceptibility. PLoS ONE, 7, e41361. doi:10.1371/journal.pone.0041361.
Sarnyai, Z. (2011). Oxytocin as a potential mediator and modulator of drug addiction. Addiction Biology, 16, 199–201. doi:10.1111/j.1369-1600.2011.00332.x.
Simons, R. L., & Klopack, E. T. (2015). Invited address: “The Times They Are A-Changin” gene expression, neuroplasticity, and developmental research. Journal of Youth and Adolescence, 44, 573–580. doi:10.1007/s10964-014-0245-1.
Simons, R. L., Lei, M K., Beach, S. S. H., Cutrona, C. E., & Philibert, R. A. (2016). Methylation of the oxytocin receptor gene mediates the effect of adversity on negative schemas and depression. Development and Psychopathology. doi:10.1017/S0954579416000420.
Tops, M., Van Peer, J. M., Korf, J., Wijers, A. A., & Tucker, D. M. (2007). Anxiety, Cortisol and attachment predict plasma oxytocin levels in healthy females. Psychophysiology, 44, 444–449. doi:10.1111/j.1469-8986.2007.00510.x.
van IJzendoorn, M. H., Belsky, J., & Bakermans-Kranenburg, M. J. (2012). Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Translational Psychiatry, 2, 1–6. doi:10.1038/tp.2012.73.
Acknowledgments
This article was supported in part by grant 5R01HD030588-16A1 and grant P30 DA027827 awarded to Gene H. Brody and by grant R01HD069439 awarded to Steven R. H. Beach. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This article was supported in part by grants 5R01HD030588-16A1 and P30 DA027827 awarded to Gene H. Brody.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants at each wave of the study (and assent was obtained from minors).
Rights and permissions
About this article
Cite this article
Beach, S.R.H., Lei, M.K., Brody, G.H. et al. Prevention of Early Substance Use Mediates, and Variation at SLC6A4 Moderates, SAAF Intervention Effects on OXTR Methylation. Prev Sci 19, 90–100 (2018). https://doi.org/10.1007/s11121-016-0709-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11121-016-0709-5