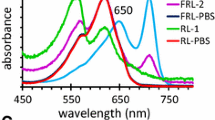
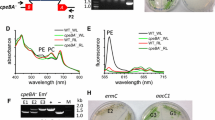
Abstract
Synechococcus ATCC 29403 (PCC 7335) is a unicellular cyanobacterium isolated from Puerto Peñasco, Sonora Mexico. This cyanobacterium performs complementary chromatic acclimation (CCA), far-red light photoacclimation (FaRLiP), and nitrogen fixation. The Synechococcus PCC 7335 genome contains at least 31 genes for proteins of the phycobilisome (PBS). Nine constitutive genes were expressed when cells were grown under white or red lights and the resulting proteins were identified by mass spectrometry in isolated PBS. Five inducible genes were expressed under white light, and phycoerythrin subunits and associated linker proteins were detected. The proteins of five inducible genes expressed under red light were identified, the induced phycocyanin subunits, two rod linkers and the rod-capping linker. The five genes for FaRLiP phycobilisomes were expressed under far-red light together with the apcF gene, and the proteins were identified by mass spectrometry after isoelectric focusing and SDS-PAGE. Based on in silico analysis, Phylogenetic trees, and the observation of a highly conserved amino acid sequence in far-red light absorbing alpha allophycoproteins encoded by FaRLiP gene cluster, we propose a new nomenclature for the genes. Based on a ratio of ApcG2/ApcG3 of six, a model with the arrangement of the allophycocyanin trimers of the core is proposed.





Similar content being viewed by others
Abbreviations
- AP:
-
Allophycocyanin
- App Mw:
-
Apparent molecular weight
- CCA:
-
Complementary chromatic acclimation
- Core-PBS:
-
Bicylindrical core containing far-red light absorbing allophycoproteins encoded by FaRLiP gene cluster (Ho et al. 2017a, b)
- DTT:
-
Dithiothreitol
- FRL:
-
Far-red light
- FaRLiP:
-
Far-red light photoacclimation
- GL:
-
Green light
- Hhc:
-
Horse heart cytochrome c
- IEF:
-
Isoelectric focusing
- LC :
-
Linker core
- LCM :
-
Linker core membrane
- LR :
-
Linker rod
- LRC :
-
Linker rod core
- PBS:
-
Phycobilisome
- PBP:
-
Phycobiliprotein
- PC:
-
Phycocyanin
- PC-PBS:
-
Phycocyanin phycobilisome
- PCB:
-
Phycocyanobilin
- PE:
-
Phycoerythrin
- PE-PBS:
-
Phycoerythrin phycobilisome
- PS:
-
Photosystem
- PMSF:
-
Phenylmethylsulfonyl fluoride
- REP:
-
Repetitive linker-like sequence
- RL:
-
Red light
- WCE:
-
Whole-cell extracts
- WL:
-
White light
References
Adir N (2005) Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynth Res 85:15–32
Anderson LK, Eiserling FA (1986) Asymmetrical core structure in phycobilisomes of the cyanobacterium Synechocystis 6701. J Mol Biol 191(3):441–451
Bryant DA, Frigaard NU (2006) Prokaryotic photosynthesis and phototrophy illuminated. Trends Microbiol 14(11):488–496
Bryant DA, Guglielmi G, Tandeau de Marsac N, Castets AM, Cohen-Bazire G (1979) The structure of the cyanobacterial phycobilisomes: a model. Arch Microbiol 123:113–127
Bryant DA, Stirewalt VL, Glauser M, Frank G, Sidler W, Zuber H (1991) A small multigene family encodes the rod-core linker polypeptides of Anabaena sp. PCC7 120 phycobilisomes. Gene 107(1):91–99
Chang L, Liu X, Li Y, Liu CC, Yang F, Zhao J, Sui SF (2015) Structural organization of an intact phycobilisome and its association with photosystem II. Cell Res 25(6):726–737
Chen M, Li Y, Birch D, Willows RD (2012) A cyanobacterium that contains chlorophyll far red absorbing photopigment. FEBS Lett 586(19):3249–3254
Cobley JG, Clark AC, Weerasurya F, Queseda SA, Xiao JY, Bandrapali N, D’Silva I, Thounaojan M, Oda JF, Sumiyoshi T, Chu MH (2002) CpeR is an activator required for expression of the phycoerythrin operon (cpeBA) in the cyanobacterium Fremyella diplosiphon and is encoded in the phycoerythrin linker polypeptide opero (cpeCDESTR). Mol Microbiol 44:1517–1531
Dong C, Tang A, Zhao J, Mullineaux CW, Shen G, Bryant DA (2009) ApcD is necessary for efficient energy transfer from phycobilisomes to photosystem I and helps to prevent photoinhibition in the cyanobacterium Synechococcus sp. PCC 7002. Biochim Biophys Acta 1787(9):1122–1128
Ducret A, Muller SA, Goldie KN, Hefti A, Sidler WA, Zuber H, Engel A (1998) Reconstitution, Characterization and mass analysis of the pentacylindrical allophycyanin core complex from the cyanobacterium Anabaena sp. PCC 7120. J Mol Biol 278:369–388
Everroad C, Six C, Partensky F, Thomas J-C, Holtzendorff J, Wood AM (2006) Biochemical bases of type IV chromatic adaptation in marine Synechococcus spp. J Bacteriol 188(9):3345–3356
Federspiel NA, Scott L (1992) Characterization of a light-regulated gene encoding a new phycoerythrin-associated linker protein from the cyanobacterium Fremyella diplosiphon. J Bacteriol 174(18):5994–5998
Gan F, Bryant DA (2015) Adaptive and acclimative responses of cyanobacteria to far-red light. Environ Microbiol 17(10):3450–3465
Gan F, Zhang S, Rockwell NC, Martin SS, Lagarias JC, Bryant DA (2014a) Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 345(6202):1312–1317
Gan F, Shen G, Bryant DA (2014b) Occurrence of far-red light photoacclimation (FaRLiP) in diverse cyanobacteria. Life 5(1):4–24
Gao X, Wei TD, Zhang N, Xie BB, Su HN, Zhang XY, Chen XL, Zhou BC, Wang ZX, Wu JW, Zhang YZ (2012) Molecular insights into the terminal energy acceptor in cyanobacterial phycobilisome. Mol Microbiol 85(5):907–915
Gindt YM, Zhou J, Bryant DA, Sauer K (1992) Core mutations of Synechococcus sp. PCC 7002 phycobilisome: a spectroscopy study. J Photochem PhotoBiol 15(1–2):75–89
Glazer AN (1984) Phycobilisome a macromolecular complex optimized for light energy transfer. Biochim Biophys Acta 768:29–51
Glazer AN (1985) Light harvesting by phycobilisomes. Ann Rev Biophys Chem 14:47–77
Glazer AN (1989) Light guides: directional energy transfer in a photosynthetic antenna. J Biol Chem 264(1):1–4
Glazer AN, Bryant DA (1975) Allophycocyanin B (λmax 671, 618 nm) a new cyanobacterial phycobiliprotein. Arch Microbiol 104:15–22
Gómez-Lojero C, Pérez-Gómez B, Krogmann DW, Peña-Diaz A (1997) The tricylindrical core of the phycobilisome of the cyanobacterium Arthrospira (Spirulina) maxima. Int. J Biochem Cell Biol 29:959–970
Gómez-Lojero C, Pérez-Gómez B, Shen G, Schluchter WM, Brayant DA (2003) Interaction of ferredoxin: NADP+ oxidoreductase with phycobilisomes and phycobilisome substructures of the cyanobacterium Synechococcus sp. strain PCC 7002. Biochemistry 42:13800–13811
Grossman AR (2003) A molecular understanding of complementary chromatic adaptation. Photosynth Res 76(1–3):207–215
Grossman AR, Schaefer MR, Chiang GG, Collier JL (1993) The phycobilisome, a light-harvesting complex responsive to environmental conditions. Microbiol Rev 57:725–749
Gutu A, Kehoe DM (2012) Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol Plant 5:1–13
Ho MY, Shen G, Canniffe DP, Zhao C, Bryant DA. (2016) Light-dependent chlorophyll f synthase is a highly divergent paralog of PsbA of photosystem II. Science 353(6302):aaf9178
Ho MY, Gan F, Shen G, Zhao C, Bryant DA (2017a) Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335: I. Regulation of FaRLiP gene expression. Photosynth Res 131(2):173–186
Ho MY, Gan F, Shen G, Bryant DA (2017b) Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335. II. Characterization of phycobiliproteins produced during acclimation to far-red light. Photosynth Res 131(2):187–202
Houmard J, Capuano V, Colombano MV, Coursin T, Tandeau de Marsac N (1990) Molecular characterization of the terminal energy acceptor of cyanobacterial phycobilisomes. Proc Natl Acad Sci USA 87(6):2152–2156
Kehoe DM, Gutu A (2006) Responding to color: the regulation of complementary chromatic adaptation. Annu Rev Plant Biol 57:127–150
Krogmann DW, Pérez-Gómez B, Gutiérrez-Cirlos EB, Chagolla-López A, Gónzalez de la Vara L, Gómez-Lojero C (2007) The presence of multidomain linkers determines the bundle-shape structure of the phycobilisome of the cyanobacterium Gloeobacter violaceus PCC 7421. Photosynth Res 93:23–27
Li Y, Lin Y, Garvey CJ, Birch D, Corkery RW, Loughlin PC, Scheer H, Willows RD, Chen M (2016) Characterization of red-shifted phycobilisomes isolated from the chlorophyll f-containing cyanobacterium Halomicronema hongdechloris. Biochim Biophys Acta 1857(1):107–114
Liu H, Zhang H, Niedzwiedzki DM, Prado M, He G, Gross ML, Blankenship RE (2013) Phycobilisomes supply excitations to both photosystems in a megacomplexes in cyanobacteria. Science 342(6162):1104–1107
Lundell DJ, Glazer AN (1981) Allophycocyanin B, a common β subunit in Synechococcus allophycocyanin B (λmax670 nm) and allophycocyanin (λmax650 nm). J Biol Chem 256:12600–12606
Lundell DJ, Yamanaka G, Glazer AN (1981) A terminal energy acceptor of the phycobilisome: the 75,000-dalton polypeptide of Synechococcus 6301 phycobilisome a new biliprotein. J Cell Biol 91:315–319
Mendoza-Hernández G, Pérez Gómez B, Krogmann DW, Gutiérrez-Cirlos EB, Gómez-Lojero C (2010) Interactions of linker proteins with the phycobiliproteins in the phycobilisome substructures of Gloeobacter violaceus. Photosynth Res 106(3):247–261
Miao D, Ding WL, Zhao BQ, Lu L, Xu QZ, Scheer H, Zhao KH (2016) Adapting photosynthesis to the near-infrared: non-covalent binding of phycocyanobilin provides an extreme spectral red-shift to phycobilisome core-membrane linker from Synechococcus sp. PCC 7335. Biochim Biophys Acta 1857(6):688–694
Mimuro M, Lipschultz CA, Gantt E (1986) Energy flow in the phycobilisome core of Nostoc sp. (MAC): two independent terminal pigments. Biochim Biophys Acta 852:307–319
Olsen MT, Nowack S, Wood JM, Becraft ED, LaButti K, Lipzen A, Martin J, Schackwitz WS, Rusch DB, Cohan FM, Bryant DA, Ward DM (2015) The molecular dimension of microbial species: 3: comparative genomics of Synechococcus strains with different light responses and in situ diel transcription patterns of associated putative ecotypes in the Mushroom Spring microbial mat. Front Microbiol 6:604
Peng PP, Dong LL, Sun YF, Zeng XL, Ding WL, Scheer H, Yang X, Zhao KH (2014) The structure of allophycocyanin B from Synechocystis PCC 6803 reveals the structural basis for the extreme redshift of the terminal emitter in phycobilisomes. Acta Crystallogr D 70:2558–2569
Pérez-Gómez B, Mendoza-Hernández G, Cabellos-Avelar T, Leyva-Castillo LE, Gutiérrez-Cirlos EB, Gómez-Lojero C (2012) A proteomic approach to the analysis of the components of the phycobilisomes from two cyanobacteria with complementary chromatic adaptation: Fremyella diplosiphon UTEX B590 and Tolypothrix PCC 7601. Photosynth Res 114(1):43–58
Reuter W, Wehrmeyer W (1990) Core structure in Mastigocladus laminosus phycobilisomes: II the central part of the tricylindrical core–APCM–contain the anchor polypeptide and no allophycocyanin B. Arch Microbiol 153:111–117
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61
Schägger H, Cramer WA, von Jagow G (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem 217:220–230
Scheer H, Zhao KH (2008) Biliprotein maturation: the chromophore attachment. Mol Microbiol 68(2):263–276
Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1(6):2856–2860
Shih PM, Wu D, Latifi A, Axen SD, Fewer DP, Talla E, Calteau A, Cai F, Tandeau de Marsac N, Rippka R, Herdman M, Sivonen K, Coursin T, Laurent T, Goodwin L, Nolan M, Davenport KW, Han CS, Rubin EM, Eisen JA, Woyke T, Gugger M, Kerfeld CA (2013) Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc Natl Acad Sci USA 110(3):1053–1058
Sidler WA (1994) The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht pp 139–216
Tandeau de Marsac N (1977) Occurrence and nature of chromatic adaptation in cyanobacteria. J Bacteriol 130(1):82–91
Tandeau de Marsac N, Houmard J (1988) Complementary chromatic adaptation: physiological conditions and action spectra. Methods Enzymol 167:318–328
Tang K, Ding W-L, Höppner A et al (2015) The terminal phycobilisome emitter, LCM: a light-harvesting pigment with a phytochrome chromophore. Proc Natl Acad Sci USA 112(52):15880–15885
Watanabe M, Ikeuchi M (2013) Phycobilisome: architecture of a light-harvesting supercomplex. Photosynth Res 116(2–3):265–276
Xu QZ, Han JX, Tang QY, Ding WL, Miao D, Zhou M, Scheer H, Zhao KH (2016) Far-red light photoacclimation: chromophorylation of FR induced α- and β-subunits of allophycocyanin from Chroococcidiopsis thermalis sp. PCC7203. Biochim Biophys Acta 1857(9):1607–1616
Yamanaka G, Glazer AN, Williams RC (1978) Cyanobacterial phycobilisomes: characterization of the phycobilisomes of Synechococcus sp. 6301. J Biol Chem 253:8303–8310
Zlenko DV, Krasilnikov PM, Stadnichuk IN (2016) Structural modeling of the phycobilisome core and its association with the photosystems. Photosynth Res 130(1–3):347–356
Zlenko DV, Galochkina TV, Krasilnikov PM, Stadnichuk IN (2017) Coupled rows of PBS cores and PSII dimers in cyanobacteria: symmetry and structure. Photosynth Res 133(1–3):245–260
Acknowledgements
This paper is dedicated to the memory of Dr. David W. Krogmann, professor, colleague, and friend. PHS received scholarship from Consejo Nacional de Ciencia y Tecnología (CONACYT, scholarship no. 261987. Dra. Emma Berta Gutiérrez-Cirlos and Dr. Diego González Halphen for his suggestions. Dr. Marco Antonio Meraz Ríos for his discussions and support in acquisition of reagents. We also thank the reviewers for the improvement of the manuscript. The authors wish to thank Mr Jorge Zarco Mendoza for his assistance to improve the technical details in the centrifugation, French press to break cells and to start the cultures. Dra. Karla Grisel Calderón-González for her technical assistance in the IEF. We also thank Mrs Leticia Gómez Sandoval for her secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herrera-Salgado, P., Leyva-Castillo, L.E., Ríos-Castro, E. et al. Complementary chromatic and far-red photoacclimations in Synechococcus ATCC 29403 (PCC 7335). I: The phycobilisomes, a proteomic approach. Photosynth Res 138, 39–56 (2018). https://doi.org/10.1007/s11120-018-0536-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-0536-6