Abstract
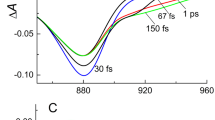
Isotropic and anisotropic pump-probe spectra of Cfx. aurantiacus chlorosomes were measured on the fs-through ps-time scales for the B798 BChl a Q y band upon direct excitation of the B798 band at T = 293 K and T = 90 K. Upon direct excitation of the B798 band, the anisotropy parameter value r(λ) was constant within the whole BChl a Q y band at any delay time at both temperatures. The value of the anisotropy parameter r decayed from r = 0.4 at both temperatures (at 200 fs delay time after excitation) to the steady-state values r = 0.1 at T = 293 K and to r = 0.09 at T = 90 K (at 30 ÷ 100 ps delay time after excitation). The results were considered within the framework of the model of uniaxial orientation distribution of BChl-a transition dipoles within a single Cfx. aurantiacus chlorosome. This implies that the B798 BChl a Q y transition dipoles, randomly distributed around the normal to the baseplate plane, form the angle θ with the plane. For this model, the theoretical dependence of the steady-state anisotropy parameter r on the angle θ was derived. According to the theoretical dependence r(θ), the angle θ corresponding to the experimental steady-state value r = 0.1 at T = 293 K was found to equal 55°. As the temperature drops to 90 K, the angle θ decreases to 54°.






Similar content being viewed by others
References
Arellano JB, Melo TB, Borrego CM, Garcia-Gil J, Naqvi KR (2000) Nanosecond laser photolysis studies of chlorosomes and artificial aggregates containing bacteriochlorophyll e: Evidence for the proximity of carotenoids and bacteriochlorophyll a in chlorosomes from Chlorobium phaeobacteroides strain CL1401. Photochem Photobiol 72:669–675
Carbonera D, Bordignon E, Giacometti G, Agostini G, Vianelli A, Vannini C (2001) Fluorescence and absorption detected magnetic resonance of chlorosomes from green bacteria Chlorobium tepidum and Chloroflexus aurantiacus. A comparative study. J Phys Chem B 105:246–255
Didraga C, Knoester J (2003) Absorption and dichroism spectra of cylindrical J aggregates and chlorosomes of green bacteria. J Lumin 102:60–66
Dracheva T, Taisova A, Fetisova Z (1998) Circular dichroism spectroscopy as a test for the chlorosome antenna structure. In: Garab G (ed) Photosynthesis: Mechanisms and Effects, vol 1. Kluwer Academic Publishers, Dordrecht, pp 129–132
Egawa A, Fujiwara T, Mizoguchi T, Kakitani Y, Koyama Y, Akutsu H (2007) Structure of the light-harvesting bacteriochlorophyll c assembly in chlorosomes from Chlorobium limicola determined by solid-state NMR. Proc Natl Acad Sci USA 104(3):790–795
Feick RG, Fitzpatrick M, Fuller RC (1982) Isolation and characterization of cytoplasmic membranes and chlorosomes from the green bacterium Chloroflexus aurantiacus. J Bacteriol 150(2):905–915
Fetisova ZG (2004) Survival strategy of photosynthetic organisms. 1. variability of the extent of light-harvesting pigment aggregation as a structural factor optimizing the function of oligomeric photosynthetic antenna (Mosk). Model Calculations Mol Biol 38:434–440
Fetisova ZG, Fok MV (1984) Optimization routes for the transformation of light energy in primary acts of photosynthesis. I. The necessity of structure optimization for photosynthetic unit and method for the calculation of its efficiency (Mosk). Mol Biol 18:1354–1359
Fetisova ZG, Kharchenko SG, Abdourakhmanov IA (1986) Strong orientational ordering of the near-infrared transition moment vectors of light-harvesting antenna bacterioviridin in chromatophores of the green photosynthetic bacterium Chlorobium limicola. FEBS Lett 199:234–236
Fetisova ZG, Freiberg AM, Timpmann KE (1988) Long-range molecular order as an efficient strategy for light harvesting in photosynthesis (London). Nature 334:633–634
Fetisova Z, Freiberg A, Mauring K, Novoderezhkin V, Taisova A, Timpmann K (1996) Excitation energy transfer in chlorosomes of green bacteria: theoretical and experimental studies. Biophys J 71:995
Frigaard N-U, Bryan D, Fetisova Z (2006) Chlorosomes: antenna organelles in green photosynthetic bacteria. In: Shively JM (ed) Complex intracellular structures in prokaryotes. Microbiology monographs, vol 2. Springer, Berlin, pp 79–114
Garab G, Van Amerongen H (2009) Linear dichroism and circular dichroism in photosynthesis research. Photosynth Res 101:135–146
Gerola PD, Olson JM (1986) A new bacteriochlorophyll a-protein complex associated with chlorosomes of green sulfur bacteria. Biochim Biophys Acta 848:69–76
Golecki JR, Oelze J (1987) Quantitative relationship between bacteriochlorophyll content, cytoplasmic membrane structure and chlorosome size in Chloroflexus aurantiacus. Arch Microbiol 148:236–241
Jendrny M, Aartsma TJ, Kohler J (2014) Insights into the Excitonic States of Individual Chlorosomes from Chlorobaculum tepidum. Biophys J 106:1921–1927
Lin S, Van Amerongen H, Struve WS (1991) Ultrafast pump-probe spectroscopy of bacteriochlorophyll c antennae in bacteriochlorophyll a-containing chlorosomes from the green photosynthetic bacterium Chloroflexus aurantiacus. Biochim Biophys Acta 1060:13–22
Linnanto JM, Korppi-Tommola JEI (2008) Investigation on chlorosomal antenna geometries: tube, lamella and spiral-type self-aggregates. Photosynth Res 96:227–245
Linnanto JM, Korppi-Tommola JEI (2013) Exciton description of chlorosome to baseplate excitation energy transfer in filamentous anoxygenic phototrophs and green sulfur bacteria. J Phys Chem B 117:11144–11161
Ma Y-Z, Cox RP, Gillbro T, Miller M (1996) Bacteriochlorophyll organization and energy transfer kinetics in chlorosomes from Chloroflexus aurantiacus depend on the light regime during growth. Photosynth Res 47:157–165
Martiskainen J, Linnanto J, Kananavičius R, Lehtovuori V, Korppi-Tommola J (2009) Excitation energy transfer in isolated chlorosomes from Chloroflexus aurantiacus. Chem Phys Lett 477:216–220
Martiskainen J, Kananavičius R, Linnanto J, Lehtivuori H, Keränen M, Aumanen V, Tkachenko N, Korppi-Tommola J (2011) Excitation energy transfer in the LHC-II trimer: from carotenoids to chlorophylls in space and time. Photosynth Res 107:195–207
Mimuro M, Hirota M, Nishimura Y, Moriyama T, Yamazaki I, Shimada K, Matsuura K (1994) Molecular organization of bacteriochlorophyll in chlorosomes of the green photosynthetic bacterium Chloroflexus aurantiacus: studies of fluorescence depolarization accompanied by energy transfer process. Photosynth Res 41:181–191
Montaňo GA, Wu HM, Lin S, Brune DC, Blankenship RE (2003) Isolation and characterization of the B798 light-harvesting baseplate from the chlorosomes of Chloroflexus aurantiacus. Biochemistry 42:10246–10251
Murata N, Fork DC (1975) Temperature dependence of chlorophyll a fluorescence in relation to the physical phase of membrane lipids in algae and higher plants. Plant Physiol 56:791–796
Niedermeier G, Shiozawa JA, Lottespeich F, Feick RG (1994) The primary structure of two chlorosome proteins from Chloroflexus aurantiacus. FEBS Lett 342:61–65
Novoderezhkin VI, Taisova AS, Fetisova ZG (1998a) Pigment organization and exciton dynamics in the B808-866 antenna of the green bacterium Chloroflexus aurantiacus. Biochem Mol Biol Intern 45:355–362
Novoderezhkin VI, Taisova AS, Fetisova ZG, Blankenship RE, Savikhin S, Buck DR, Struve WS (1998b) Energy transfers in the B808-866 antenna from the green bacterium Chloroflexus aurantiacus. Biophys J 74:2069–2075
Novoderezhkin VI, Taisova AS, Fetisova ZG (2001) Unit building block of the oligomeric chlorosomal antenna of the green photosynthetic bacterium Chloroflexus aurantiacus: modeling of nonlinear optical spectra. Chem Phys Lett 335:234–240
Novoderezhkin V, van Grondelle R (2002) Exciton-vibrational relaxation and transient absorption dynamics in LH1 of Rhodopseudomonas viridis: a redfield theory approach. J Phys Chem B 106:6025–6037
Oelze J (1992) Light and oxygen regulation of the synthesis of bacteriochlorophyll a and bacteriochlorophyll c in Chloroflexus aurantiacus. J Bacteriol 174:5021–5026
Oelze J, Fuller RC (1983) Temperature dependence of growth and membrane-bound activities of Chloroflexus aurantiacus energy metabolism. J Bacteriol 155:90–96
Oelze J, Fuller RC (1987) Growth rate and control of development of the photosynthetic apparatus in Chloroflexus aurantiacus. Arch Microbiol 148:132–136
Oelze J, Golecki JR (1995) Membranes and chlorosomes of green bacteria: structure, composition and development. In: Blankenship RE, Madigan MT, Bauer CE (eds) Anoxygenic Photosynthetic Bacteria. Kluwer Academic Publishers, The Netherlands, pp 259–278
Olson JM (1980) Chlorophyll organization in green photosynthetic bacteria. Biochim Biophys Acta 594:33–51
Olson JM (1998) Chlorophyll organization and function in green photosynthetic bacteria. Photochem Photobiol 67:61–75
Oostergetel GT, van Amerongen H, Boekema EJ (2010) The chlorosome: a prototype for efficient light harvesting in photosynthesis. Photosynth Res 104(2–3):245–255
Overath P, Thilo L, Triuble H (1976) Lipid phase transitions and membrane function. Trends Biochem Sci 1:186–189
Pedersen MØ, Linnanto J, Frigaard NU, Nielsen NC, Miller M (2010) A model of the protein-pigment baseplate complex in chlorosomes of photosynthetic green bacteria. Photosynth Res 104:233–243
Pierson BK, Castenholz RW (1992) The family Chloroflexaceae. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds) The Prokaryotes, vol 4, 2nd edn., Springer-Verlag, NY, Heidelberg, pp 3754–3774
Pierson BK, Castenholz RW (1995) Taxonomy and physiology of filamentous anoxygenic phototrophs. In: Blankenship RE, Madigan MT, Bauer CE (eds) Anoxygenic Photosynthetic Bacteria. Kluwer Academic Publishers, Dordrecht, pp 31–47
Pšenčik J, Ikonen TP, Laurinmäki P, Merckel MC, Butcher SJ, Serimaa RE, Tuma R (2004) Lamellar organization of pigments in chlorosomes, the light harvesting system of green bacteria. Biophys J 87:1165–1172
Pšenčik J, Arellano JB, Ikonen TP, Borrego CM, Laurinmäki P, Butcher SJ, Serimaa RE, Tuma R (2006) Internal structure of chlorosomes from brown-colored Chlorobium species and the role of carotenoids in their assembly. Biophys J 91:1433–1440
Pšenčik J, Collins AM, Liljeroos L, Torkkeli M, Laurinmäki P, Ansink HM, Ikonen TP, Serimaa RE, Blankenship RE, Tuma R, Butcher SJ (2009) Structure of chlorosomes from the green filamentous bacterium Chloroflexus aurantiacus. J Bacteriol 191:6701–6708
Pšenčik J, Torkkeli M, Zupčanová A, Vácha F, Serimaa RE, Tuma R (2010) The lamellar spacing in self-assembling bacteriochlorophyll aggregates is proportional to the length of the esterifying alcohol. Photosynth Res 104:211–219
Saga Y, Tamiaki H (2006) Transmission electron microscopic study on supramolecular nanostructures of bacteriochlorophyll self-aggregates in chlorosomes of green photosynthetic bacteria. J Biosc Bioeng 102:18–23
Saga Y, Wazawa T, Mizoguchi T, Ishii Y, Yanagida T, Tamiaki H (2002) Spectral heterogeneity in single light-harvesting chlorosomes from green sulfur photosynthetic bacterium Chlorobium tepidum. Photochem Photobiol 75:433–436
Sakuragi Y, Frigaard N-U, Shimada K, Matsuura K (1999) Association of bacteriochlorophyll a with the CsmA protein in chlorosomes of the photosynthetic green filamentous bacterium Chloroflexus aurantiacus. Biochim Biophys Acta 1413:172–180
Savikhin S, Buck DR, Struve WS, Blankenship RE, Taisova AS, Novoderezhkin VI, Fetisova ZG (1998) Exciton delocalization in the bacteriochlorophyll c antenna of the green bacterium Chloroflexus aurantiacus as revealed by ultrafast pump-probe spectroscopy. FEBS Lett 430:323–326
Schmidt K, Maarzahl M, Mayer F (1980) Development and pigmentation of chlorosomes in Chloroflexus aurantiacus Ok-70-fl. Arch Microbiol 127:87–97
Shibata Y, Saga Y, Tamiaki H, Itoh S (2006) Low temperature fluorescence from single chlorosomes, photosynthetic antenna complexes of green filamentous and sulfur bacteria. Biophys J 91:3787–3796
Shibata Y, Saga Y, Tamiaki H, Itoh S (2007) Polarized fluorescence of aggregated bacteriochlorophyll c and baseplate bacteriochlorophyll a in single chlorosomes isolated from Chloroflexus aurantiacus. Biochemistry 46:7062–7068
Shibata Y, Saga Y, Tamiaki H, Itoh S (2009) Anisotropic distribution of emitting transition dipoles in chlorosome from Chlorobium tepidum: fluorescence polarization anisotropy study of single chlorosomes. Photosynth Res 100:67–78
Shibata Y, Tateishi S, Nakabayashi S, Itoh S, Tamiaki H (2010) Intensity borrowing via excitonic couplings among Soret and Qy-transitions of bacteriochlorophylls in the pigment aggregates of chlorosomes, the light-harvesting antennae of green sulfur bacteria. Biochemistry 49:7504–7515
Somsen OJ, van Grondelle R, van Amerongen H (1996) Spectral broadening of interacting pigments: polarized absorption by photosynthetic proteins. Biophys J 71(4):1934–1951
Sprague SD, Varga AR (1986) Membrane architecture of anoxygenic photosynthetic bacteria. In: Staehelin LA, Arntzen CJ (eds) Encyclopedia of Plant Physiology, vol 19. Springer - Verlag, Berlin-Heidelberg, pp 603–619
Sprague SG, Staehelin LA, DiBartolomeis MJ, Fuller RC (1981) Isolation and development of chlorosomes in the green bacterium Chloroflexus aurantiacus. J Bacteriol 147:1021–1031
Staehelin LA, Golecki JR, Drews G (1978) Visualization of the supramolecular architecture of chlorosomes (Chlorobium type vesicles) in freeze-fractured cells of Chloroflexus aurantiacus. Arch Microbiol 119:269–277
Staehelin LA, Golecki JR, Drews G (1980) Supramolecular organization of chlorosomes (Chlorobium Vesicles) and of their membrane attachment sites in Chlorobium limicola. Biochim Biophys Acta 589:30–45
Taisova AS, Keppen OI, Lukashev EP, Arutyunyan AM, Fetisova ZG (2002) Study of the chlorosomal antenna of the green mesophilic filamentous bacterium Oscillochloris trichoides. Photosynth Res 74:73–85
Taisova AS, Lukashev EP, Fedorova NV, Zobova AV, Dolgova TA, Fetisova ZG (2012) Experimental proof of optimality of interfacing of chlorosome BChl c and membrane BChl a subantennae in superantenna of photosynthetic green bacteria from the Oscillochloridaceae family. Dokl Biochem Biophys 444:154–157
Taisova AS, Yakovlev AG, Fetisova ZG (2014) Size variability of the unit building block of peripheral light-harvesting antennas as a strategy for effective functioning of antennas of variable size that is controlled in vivo by light intensity. Biochemistry (Mosc) 79:251–259
Van Amerongen H, Vasmel H, van Grondelle R (1988) Linear dichroism of chlorosomes from Chloroflexus aurantiacus in compressed gels and electric fields. Biophys J 54:65–76
Van Amerongen H, van Haeringen B, van Gurp M, van Grondellle R (1991) Polarized fluorescence measurements on ordered photosynthetic antenna complexes. Biophys J 59:992–1001
Van Dorssen RJ, Amesz J (1988) Pigment organization and energy transfer in the green photosynthetic bacterium Chloroflexus aurantiacus. III. Energy transfer in whole cells. Photosynth Res 15:177–189
Van Dorssen RJ, Vasmel H, Amesz J (1986) Pigment organization and energy transfer in the green photosynthetic bacterium Chloroflexus aurantiacus. II. The chlorosome. Photosynth Res 9:33–45
Vasmel H, van Dorssen RJ, De Vos GJ, Amesz J (1986) Pigment organization and energy transfer in the green photosynthetic bacterium Chloroflexus aurantiacus: I. The cytoplasmic membrane. Photosynth Res 7:281–294
Pierson BK, Castenholz RW (I974) Pigments and growth in Chloroflexus aurantiacus, a phototrophic filamentous bacterium. Arch Microbiol 100: 283–305
Yakovlev AG, Shkuropatov AY, Shuvalov VA (2000) Nuclear wavepacket motion producing a reversible charge separation in bacterial reaction centers. FEBS Lett 466:209–212
Yakovlev AG, Novoderezhkin VI, Taisova AS, Fetisova ZG (2002a) Exciton dynamics in the chlorosomal antenna of the green bacterium Chloroflexus aurantiacus: experimental and theoretical studies of femtosecond pump-probe spectra. Photosynth Res 71:19–32
Yakovlev AG, Taisova AS, Fetisova ZG (2002b) Light control over the size of an antenna unit building block as an effecient strategy for light harvesting in photosynthesis. FEBS Lett 512:129–132
Yakovlev A, Novoderezhkin V, Taisova A Zobova A, Fetisova Z (2013) Optimal mutual orientational ordering of QY transition dipoles of adjacent subantennae pigments in the superantenna of the photosynthetic green bacterium Chloroflexus aurantiacus. Theoretical and experimental studies. In: Kuang T, Lu C, Zhang L (eds) Photosynthesis Research for Food, Fuel and Future, Advanced Topics in Science and Technology in China-15th International Conference on Photosynthesis, Symposium 03: Light Harvesting Anaerobic Systems. Zhejiang University Press, Heidelberg, pp 113–116
Zobova AV, Yakovlev AG, Taisova AS, Fetisova ZG (2009) The search for an optimal orientational ordering of Qy transition dipoles of subantennae molecules in the superantenna of photosynthetic green bacteria: model calculations. Molecular Biology (Mosk) 43:426–443
Zobova A, Taisova A, Lukashev E, Fedorova N, Baratova L, Fetisova Z (2011) CsmA protein is associated with BChl a in the baseplate subantenna of chlorosomes of the photosynthetic green filamentous bacterium Oscillochloris trichoides belonging to the family Oscillochloridaceae. J of Biophysics. doi:10.1155/2011/860382
Acknowledgments
The work was supported by the Russian Foundation for Basic Research (Grant 08-04-01587a, 10-04-01758-a).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yakovlev, A., Novoderezhkin, V., Taisova, A. et al. Orientation of B798 BChl a Q y transition dipoles in Chloroflexus aurantiacus chlorosomes: polarized transient absorption spectroscopy studies. Photosynth Res 125, 31–42 (2015). https://doi.org/10.1007/s11120-014-0060-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0060-2