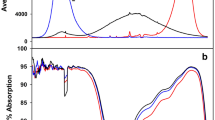
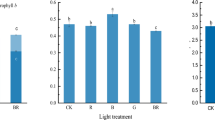
Abstract
The regulatory effect of light quality on the photosynthetic apparatus in attached leaves of rice plants was investigated by keeping rice plants under natural light, in complete darkness, or under illumination with light of different colors. When leaves were left in darkness and far-red (FR)-light conditions for 6 days at 30°C, there was an initial lag in chlorophyll (Chl) content, Chl a/b ratio, and maximum photosystem (PS) II photochemistry that lasted until the second day; these then rapidly decreased on the fourth day. In contrast, ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) rapidly disappeared with no lag under low or zero light conditions. By using spectrophotometric quantitation, it was determined that the PSII and PSI reaction centers were regulated by light quality, but cytochrome (Cyt) f was regulated by light intensity. However, the PSII heterogeneity was also strongly modified by the light intensity; PSIIα with the large antenna decreased markedly both in content and in antenna size. Consequently, the PSIIα/PSI ratio declined under FR-light because the low intensity of FR-light dominated over its quality in the modulation of the PSIIα/PSI ratio. An imbalance between them induced the generation of reactive oxygen species (ROS), although the ROS were scavenged by stromal enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR). The activities of these stromal enzymes are also regulated by light quality. Thus, although the photosynthetic apparatus is regulated differently depending on light quality, light quality may play an important role in the regulation of the photosynthetic apparatus.


Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- C-550:
-
Electrochromic band shift of pheophytin in the PSII reaction center complex
- Chl:
-
Chlorophyll
- Cyt:
-
Cytochrome
- DCMU:
-
3-(3,4-Dichlorophenyl)-1,1-dimethylurea
- Fv/Fm:
-
Maximal photochemical efficiency of PSII in the dark-adapted leaves
- GR:
-
Glutathione reductase
- GSH and GSSG:
-
Reduced and oxidized glutathione, respectively
- PS:
-
Photosystem
- QA :
-
Primary quinone acceptor in PSII
- QB :
-
Secondary quinone acceptor in PSII
- R- and FR-light:
-
Red and far-red light, respectively
- ROS:
-
Reactive oxygen species
- Rubisco:
-
Ribulose 1,5-bisphosphate carboxylase/oxygenase
- SOD:
-
Superoxide dismutase
References
Allen JF (1995) Thylakoid protein phosphorylation, state 1-state 2 transitions, and photosystem stoichiometry adjustment: redox control at multiple levels of gene expression. Physiol Plant 93:196–205
Amako K, Chen GX, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Anderson JM (1986) Photoregulation of the composition, function, and structure of thylakoid membranes. Annu Rev Plant Physiol 37:93–136
Anderson JM, Chow WS, Park Y-I (1995) The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46:129–139
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Beyer WF, Fridovich I (1987) Assaying for superoxide activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Buchanan-Wollaston V (1997) The molecular biology of leaf senescence. J Exp Bot 48:181–199
Chow WS, Anderson JM (1987) Photosynthetic responses of Pisum sativum to an increase in irradiance during growth 1. Photosynthetic activities. Aust J Plant Physiol 14:1–8
Chow WS, Anderson JM, Melis A (1990a) The photosystem stoichiometry in thylakoids of some Australian shade-adapted plant species. Aust J Plant Physiol 17:665–674
Chow WS, Melis A, Anderson JM (1990b) Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci USA 87:7502–7506
De la Torre WR, Burkey KO (1990) Acclimation of barley to changes in light intensity: photosynthetic electron transport activity and components. Photosynth Res 24:127–136
del Río LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jiménez A, López-Huertas E, Hernández JA (1998) The activated oxygen role of peroxisomes in senescence. Plant Physiol 116:1195–1200
El Bissati K, Kirilovsky D (2001) Regulation of psbA and psaE expression by light quality in Synechocystis species PCC 6803. A redox control mechanism. Plant Physiol 125:1988–2000
Evans JR (1987) The relationship between electron transport components and photosynthetic capacity in pea leaves grown at different irradiances. Aust J Plant Physiol 14:157–170
Hikosaka K (1996) Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Planta 198:144–150
Jablonski PP, Anderson JW (1978) Light dependent reduction of oxidised glutathione by ruptured chloroplasts. Plant Physiol 61:221–225
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leong T-Y, Anderson JM (1984) Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. 2. Regulation of electron transport capacities, electron carriers, coupling factor (CF1) activity and rates of photosynthesis. Photosynth Res 5:117–128
McCauley SW, Melis A (1986) Quantitation of photosystem II in spinach chloroplasts. Biochim Biophys Acta 849:175–182
Melis A (1991) Dynamics of photosynthetic membrane composition and function, Biochim. Biophys Acta 1058:87–106
Melis A (1996) Excitation energy transfer: functional and dynamic aspects of Lhc (cab) proteins. In: Ort DR, Yocum CF (eds) Advances in photosynthesis, vol 4. Oxygenic photosynthesis: the light reactions. Kluwer, Dordrecht, pp 523–538
Melis A, Homann PH (1975) Kinetic analysis of the fluorescence induction in 3-(3,4-dichlorophenyl)-1,1-dimethylurea poisoned chloroplasts. Photochem Photobiol 21:431–437
Melis A, Homann PH (1976) Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol 23:343–350
Mishra D, Pradhan P (1973) Regulation of senescence in detached rice leaves by light, benzimidazole and kinetin. Exp Gerontol 8:153–155
Monsi M, Saeki T (1953) Über den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung für die Stoffproduction. Jpn J Bot 14:22–52
Mullineaux P, Ball L, Escobar C, Karpinska B, Creissen G, Karpinski S (2000) Are diverse signalling pathways integrated in the regulation of Arabidopsis antioxidant defence gene expression in response to excess excitation energy? Phil Trans R Soc Lond B 355:1531–1540
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Okada K, Katoh S (1998) Two long-term effects of light that control the stability of proteins related to photosynthesis during senescence of rice leaves. Plant Cell Physiol 39:394–404
Okada K, Inoue Y, Satoh K, Katoh S (1992) Effects of light on degradation of chlorophyll and proteins during senescence of detached rice leaves. Plant Cell Physiol 33:1183–1191
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Rousseaux MC, Hall AJ, Sánchez RA (1996) Far-red enrichment and photosynthetically active radiation level influence leaf senescence in field-grown sunflower. Physiol Plant 96:217–224
Singh AK, Bhattacharyya-Pakrasi M, Elvitigala T, Ghosh B, Aurora R, Pakrasi HB (2009) A systems-level analysis of the effects of light quality on the metabolism of a cyanobacterium. Plant Physiol 151:1596–1608
Smith H, Samson G, Fork DC (1993) Photosynthetic acclimation to shade: probing the role of phytochromes using photomorphogenic mutants of tomato. Plant Cell Environ 16:929–937
Thimann KV, Tetley RM, Krivak BM (1977) Metabolism of oat leaves during senescence. V. Senescence in light. Plant Physiol 59:448–454
Thompson JE, Ledge RL, Barber RF (1987) The role of free radicals in senescence and wounding. New Phytol 105:317–344
Tinoco-Ojanguren C, Pearcy RW (1995) A comparison of light quality and quantity effects on the growth and steady-state and dynamic photosynthetic characteristics of three tropical tree species. Funct Ecol 9:222–230
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Wang H, Gu M, Cui J, Shi K, Zhou Y, Yu J (2009) Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J Photochem Photobiol B 96:30–37
Yamazaki J, Kamimura Y (2002) Relationship between photosystem stoichiometries and changes in active oxygen scavenging enzymes in natural grown rice seedlings. Plant Growth Regul 36:113–120
Yamazaki J, Kamimura Y, Okada M, Sugimura Y (1999a) Changes in photosynthetic characteristics and photosystem stoichiometries in the lower leaves in rice seedlings. Plant Sci 148:155–163
Yamazaki J, Kamimura Y, Sugimura Y (1999b) Changes in photosynthetic apparatus in the juvenile rice canopy and a possible function of photosystem I in the bottom leaves. Z Naturforsch 54c:915–922
Yamazaki J, Kamimura Y, Nakayama K, Okada M, Sugimura Y (2000) Effects of light on the photosynthetic apparatus and a novel type of degradation of the photosystem I peripheral antenna complexes under darkness. J Photochem Photobiol B 55:37–42
Yamazaki J, Yoda E, Takahashi A, Sonoike K, Maruta E (2007) Pacific Ocean and Japan Sea ecotypes of Japanese beech (Fagus crenata) differ in photosystem responses to continuous high light. Tree Physiol 27:961–968
Acknowledgments
I would like to express my gratitude to my mentors, Professor Yasumaro Kamimura and Professor Emiko Maruta, for their many critical suggestions and for their encouragement throughout this study. I also thank Tomoyuki Nakayama, Junko Sakabe, Tomomi Shinobu, Reina Arakawa, Takeshi Katoh, Sachiko Mizukami, Ryoko Yabe, Mayo Karube, Yuko Shinomiya, Eriko Tsurumi, and my other collaborators for their excellent technical assistance and many suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamazaki, Jy. Is light quality involved in the regulation of the photosynthetic apparatus in attached rice leaves?. Photosynth Res 105, 63–71 (2010). https://doi.org/10.1007/s11120-010-9567-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-010-9567-3