Abstract
Ferredoxin:NADP+ oxidoreductase is an enzyme associated with the stromal side of the thylakoid membrane in the chloroplast. It is involved in photosynthetic linear electron transport to produce NADPH and is supposed to play a role in cyclic electron transfer, generating a transmembrane pH gradient allowing ATP production, if photosystem II is non-functional or no NADP+ is available for reduction. Different FNR isoforms have been described in non-photosynthetic tissues, where the enzyme catalyses the NADPH-dependent reduction of ferredoxin (Fd), necessary for some biosynthetic pathways. Here, we report the isolation and purification of two FNR isoproteins from wheat leaves, called FNR-A and FNR-B. These forms of the enzyme were identified as products of two different genes, as confirmed by mass spectrometry. The molecular masses of FNR-A and FNR-B were 34.3 kDa and 35.5 kDa, respectively. The isoelectric point of both FNR-A and FNR-B was about 5, but FNR-B appeared more acidic (of about 0.2 pH unit) than FNR-A. Both isoenzymes were able to catalyse a NADPH-dependent reduction of dibromothymoquinone and the mixture of isoforms catalysed reduction of cytochrome c in the presence of Fd. For the first time, the pH- and ionic strength dependent oligomerization of FNRs is observed. No other protein was necessary for complex formation. The putative role of the two FNR isoforms in photosynthesis is discussed based on current knowledge of electron transport in chloroplasts.










Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- DBMIB:
-
Dibromothymoquinone
- Cyt b6f:
-
Cytochrome b6f complex
- FAD:
-
Flavin adenine dinucleotide
- FA:
-
Formic acid
- Fd:
-
Ferredoxin
- FNR:
-
Ferredoxin:NADP+ oxidoreductase
- NADP:
-
Nicotinamide adenine dinucleotide phosphate
References
Apley EC, Wagner E, Engelbrecht S (1985) Rapid procedure for the preparation of ferredoxin-NADP+ oxidoreductase in molecularly pure form at 36 kilodaltons. Anal Biochem 150:145–154
Arakaki AK, Ceccarelli EA, Carrillo N (1997) Plant-type ferredoxin-NADP+ reductase: A basal structural framework and a multipily of functions. FASEB J 11:133–140
Arnon DI, Chain RK (1975) Regulation of ferredoxin-catalysed photosynthetic phosphorylations. Proc Natl Acad Sci USA 72:4961–4965
Avron M, Jegendorf AT (1956) A TPNH diaphorase from chloroplast. Arch Biochem Biophys 65:475–490
Bates PA, Kelley LA, MacCallum RM et al (2001) Enhancement of protein modelling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM, Proteins: structure, function and genetics. Suppl 5:39–46
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Bojko M, Więckowski S (1995) Diaphorase activity of ferredoxin:NADP+ oxidoreductase in the presence of dibromothymoquinone. Phytochemistry 40:661–665
Bojko M, Więckowski S (2001) Three substrate binding site on spinach ferredoxin:NADP+ oxidoreductase. Studies with selectively acting inhibitors. Photosynthetica 39:553–556
Bojko M, Kruk J, Więckowski S (2003) Plastoquinones are effectively reduced by ferredoxin:NADP+ oxidoreductase in the presence of sodium cholate micelles. Significance for cyclic electron transport and chlororespiraion. Phytochemistry 64:1055–1060
Bowsher CD, Fletcher GJ (2001) Ferredoxin:NADP+ oxidoreductase regulation in developing wheat leaf. In Proceedings of the 12th International Congress on Photosynthesis, Brisbane Convention & Exhibition Centre, Queensland, Australia, August 18–23
Bruns CM, Karplus PA (1995) Refined crystal structure of spinach ferredoxin reductase at 1.7 A resolution: oxidized, reduced and 2′-phospho-5′-AMP bound states. J Mol Biol 247:125–145
Carrillo N, Ceccarelli EA (2003) Open questions in ferredoxin-NADP+ reductase catalytic mechanism. Eur J Biochem 270:1900–1915
Chen YP, Yoch DC (1989) Isolation, characterisation and biological activity of ferredoxin-NAD+ reductase from the methane oxidizer Methylosinus trichosporium OB3b. J Bacteriol 171:5012–5016
Corneille S, Cournac L, Guedeney G et al (1998) Reduction of the plastoquinone pool by exogenous NADH and NADPH in higher plant chloroplasts. Characterization of a NAD(P)H-plastoquinone oxidoreductase activity. Biochim Biophys Acta 1363:59–69
Emanuelsson O, Nielsen H, von Heijne G (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Prot Sci 8:978–984
Garnier J, Gibrat J-F, Robson B (1996) GOR secondary structure prediction method version IV. In: Doolittle RF (ed) Methods in enzymology, vol 266, pp 540–553
Gasteiger E, Hoogland C, Gattiker A et al (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook, Humana Press, pp 571–607
Green LS, Yee BC, Buchanan BB et al (1991) Ferredoxin and ferredoxin-NADP+ reductase from photosynthetic and nonphotosynthetic tissues of tomato. Plant Physiol 96:1207–1213
Greenfield N, Fasman GD (1969) Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 8:4108–4116
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hanke GT, Okutani S, Satomi Y et al (2005) Multiple iso-proteins of FNR in Arabidopsis: evidence for different contributions to chloroplast function and nitrogen assimilation. Plant Cell Environ 28:1146–1157
Hasumi H, Nagata E, Nakamura S (1983) Molecular heterogenity of ferredoxin:NADP+ reductase from spinach leaves. Biochem Biophys Res Commun 110:280–286
Heldt HW, Werdan K, Milovancev M et al (1973) Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta 314:224–241
Hoagland DR, Arnon DI (1938) The water culture method for growing plants without soil. Calif Agric Exp Stn Bull 347:36–39
Hodges M, Miginiac-Maslow M (1993) The in vitro effects of ATP and protein phosphorylation on the activity of ferredoxin: NADP oxidoreductase from spinach chloroplasts. Plant Sci 90:21–29
Hosler JP, Yocum CF (1987) Regulation of cyclic photophosphorylation during ferredoxin-mediated electron transport. Plant Physiol 83:965–969
Jin T, Morigasaki S, Wada K (1994) Purification and characterisation of two ferredoxin-NADP+ oxidoreductase isoforms from the first foliage leaves of mung bean (Vigna radiata) seedlings. Plant Physiol 106:697–702
Kamińska J, Dzięcioł J, Kościelak J (1999) Triazine dyes as inhibitors and affnity ligands of glycosyltransferases. Glycoconjugate J 16:719–723
Kontopidis G, Holt C, Sawyer L (2002) The ligand-binding site of bovine β-lactoglobulin: evidence for a function? J Mol Biol 318:1043–1055
Kramer DM, Sacksteder CA, Cruz JA (1999) How acidic is the lumen? Photosynth Res 60:151–163
Krapp AR, Rodriguez RE, Poli HO et al (2002) The flavoenzyme ferredoxin (flavodoxin)-NADP(H) reductase modulates NADP(H) homeostasis during the soxRS response of Escherichia coli. J Bacteriol 5:1474–1480
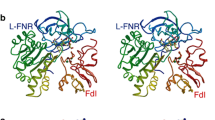
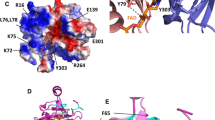
Kurisu G, Kusunoki M, Katoh E et al (2001) Structure of the electron transfer complex between ferredoxin and ferredoxin-NADP+ reductase. Nat Struct Biol 8:117–121
Laemmli U K (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lintala M, Allahverdiyeva Y, Kidron H et al (2007) Structural and functional characterization of ferredoxin-NADP+-oxidoreductase using knock-out mutants of Arabidopsis. Plant J 49:1041–1052
Maeda M, Lee YH, Ikegami T et al (2005) Identification of the N- and C-terminal substrate binding segments of ferredoxin-NADP+ reductase by NMR. Biochemistry 44:10644–10653
Matthijs HC, Coughlan SJ, Hind G (1986) Removal of ferredoxin:NADP+ oxidoreductase from thylakoid membranes, rebinding to depleted membranes, and identification of the binding site. J Biol Chem 261:12154–12158
Milani M, Balconi E, Aliverti A et al (2007) Ferredoxin-NADP+ reductase from Plasmodium falciparum undergoes NADP+-dependent dimerization and inactivation: functional and crystallographic analysis. J Mol Biol 367:501–513
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Okutani S, Hanke GT, Satomi Y et al (2005) Three maize leaf ferredoxin:NADPH oxidoreductases vary in subchloroplast location, expression, and interaction with ferredoxin. Plant Physiol 139:1451–1459
Onda Y, Matsumura T, Kimata-Ariga Y et al (2000) Differential interaction of maize root ferredoxin:NADP(+) oxidoreductase with photosynthetic and non-photosynthetic ferredoxin isoproteins. Plant Physiol 123:1037–1045
Pasteur N, Peasteur G, Bonhomme F et al (1988) Practical isozyme genetics. Ellis Horwood Limited Publishers. Chichester. Halsted Press: a division of John Willey & Sons New York-Chichester-Brisbane-Toronto
Perkins DN, Pappin DJ, Creasy DM et al (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551–3567
Pessino S, Caelles C, Puigdomenech P et al (1994) Structure and characterization of the gene encoding the ferredoxin-NADP reductase-binding protein from Zea mays L. Gene 147:205–208
Prasad GS, Kresge N, Muhlberg AB et al (1998) The crystal structure of NADPH:Ferredoxin reductase from Azotobacter vinelandii. Prot Sci 7:2541–2549
Razquin P, Fillat MF, Schmitz S et al (1996) Expression of ferredoxin-NADP+ reductase in heterocysts from Anabaena sp. Biochem J 316:157–160
Rodriguez RE, Lodeyro A, Poli HO et al (2007) Transgenic tobacco plants overexpressing chloroplastic ferredoxin-NADP(H) reductase display normal rates of photosynthesis and increased tolerance to oxidative stress. Plant Physiol 143:639–649
Shin M (2004) How is ferredoxin-NADP reductase involved in the NADP photoreduction of chloroplast? Photosynth Res 80:307–313
Shin M, Ishida H, Nozaki Y (1985) A new protein factor, connectein as a constituent of the large form of ferredoxin-NADP reductase. Plant Cell Physiol 26:559–563
van Thor JJ, Geerlings TH, Matthijs HC et al (1999) Kinetic evidence for the PsaE dependent transient ternary complex photosystem I/Ferredoxin/Ferredoxin:NADP+ reductase in a cyanobacterium. Biochemistry 38:12735–12746
van Thor JJ, Jeanjean R, Havaux M et al (2002) Salt shock-inducible photosystem I cyclic electron transfer in Synechocystis PCC6803 relies on binding of ferredoxin:NADP+ reductase to the thylakoid membranes via its CpcD phycobilisome-linker homologous N terminal domain. Biochim Biophys Acta 1457:129–144
Weber N, Strotmann H (1993) On the function of subunit PsaE in chloroplast Photosystem I. Biochim Biophys Acta 1143:204–210
Zanetti G, Cidaria D, Curti B (1982) Preparation of apoprotein from spinach ferredoxin-NADP+ reductase. Studies on the resolution process and characterization of the FAD reconstituted holoenzyme. Eur J Biochem 126:453–458
Zhang H, Whitelegge JP, Cramer WA (2001) Ferredoxin:NADP+ oxidoreductase is a subunit of then chloroplast cytochrome b6f complex. J Biol Chem 276:38159–38165
Acknowledgements
The authors are grateful to Dr. Maciej Kotlinski from Department of Plant Molecular Biology, Warsaw University, Miecznikowa 1, PL-02-096 Warsaw, Poland and Dr. Adam Jagielski for help in MS analysis. The authors also would like to thank Dr. Andrzej Waloszek for his critical reading of the manuscript. This work was financed from the budget of the Polish Ministry of Science for the years 2005–2008 under project No: 2P04A06328.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grzyb, J., Malec, P., Rumak, I. et al. Two isoforms of ferredoxin:NADP+ oxidoreductase from wheat leaves: purification and initial biochemical characterization. Photosynth Res 96, 99–112 (2008). https://doi.org/10.1007/s11120-008-9289-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-008-9289-y