Abstract
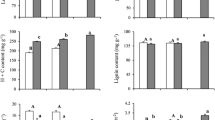
We combined measurements of short-term (during gas exchange) and long-term (from plant dry matter) carbon isotope discrimination to estimate CO2 leakiness from bundle sheath cells in six C4 species (three grasses and three dicots) as a function of leaf insertion level, growth temperature and short-term irradiance. The two methods for determining leakiness yielded similar results (P > 0.05) for all species except Setaria macrostachya, which may be explained by the leaf of this species not being accommodating to gas exchange. Leaf insertion level had no effect on leakiness. At the highest growth temperature (36°C) leakiness was lower than at the two lower growth temperatures (16°C and 26°C), between which no differences in leakiness were apparent. Higher irradiance decreased leakiness in three species, while it had no significant effect on the others (there was an opposite trend in two species). The inverse response to increasing irradiance was most marked in the two NAD-ME dicots (both Amaranthus species), which both showed almost 50% leakiness at low light (300 μmol quanta m−2 s−1) compared to about 30% at high light (1,600 μmol quanta m−2 s−1). NADP-ME subtype grasses had lower leakiness than NAD-ME dicots. Although there were exceptions, particularly in the effect of irradiance on leakiness in Sorghum and Boerhavia, we conclude that conditions favourable to C4 photosynthesis (high temperature and high light) lead to a reduction in leakiness.



Similar content being viewed by others
Abbreviations
- δ13C:
-
13C/12C composition of sample expressed as (‰) difference against standard V-PDB
- Δ:
-
Carbon isotope discrimination (usually in ‰)
- φ:
-
CO2 leakiness of bundle sheath cells (%)
- CA:
-
Carbonic anhydrase
- MC:
-
Mesophyll cells
- BSC:
-
Bundle sheath cells
- PEPC:
-
Phosphoenolpyruvate carboxylase
- Rubisco:
-
Ribulose 1,5-bisphosphate carboxylase/oxygenase
References
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Brown WV (1975) Variations in anatomy, associations and origins of kranz tissue. Am J Bot 62:395–402
Brown RH, Byrd GT (1993) Estimation of bundle sheath cell conductance in C4 species and O2 insensitivity of photosynthesis. Plant Physiol 103:1183–1188
von Caemmerer S, Furbank RT (1999) Modelling C4 photosynthesis. In: Sage RF, Monson RK (eds) C4 plants biology. Academic Press, London, pp 173–211
von Caemmerer S, Furbank RT (2003) The C4 pathway: an efficient CO2 pump. Photosynth Res 77:191–207
von Caemmerer S, Millgate A, Farquhar GD, Furbank RT (1997) Reduction of Rubisco by antisense RNA in C4 plant Flaveria bidentis leads to reduced assimilation rates and increased carbon isotope discrimination. Plant Physiol 113:469–477
Cousins AB, Badger MR, von Caemmerer S (2006) Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis. Insight from antisense RNA in Flaveria bidentis. Plant Physiol 141:232–242
Dai Z, Ku MSB, Edwards GE (1995) C4 photosynthesis – the effects of leaf development on CO2 concentrating mechanism and photorespiration in maize. Plant Physiol 107:815–825
Dengler NG, Nelson T (1999) Leaf structure and development in C4 plants. In: Sage RF, Monson RK (eds) C4 plants biology. Academic Press, London, pp 133–172
Dwyer SA, Ghannoum O, Nicotra A, von Caemmerer S (2007) High temperature acclimation of C4 photosynthesis is linked to changes in photosynthetic biochemistry. Plant Cell Env 30:53–66
Espelie KE, Kolattukudy PE (1979) Composition of aliphatic components of suberin from the bundle sheaths of Zea mays leaves. Plant Sci Lett 15:225–230
Ehleringer J, Pearcy RW (1983) Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiol 73:555–559
Evans RJ, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13:281–292
Farquhar GD (1983) On the nature of carbon isotope discrimination in C4 species. Aust J Plant Physiol 10:205–226
Fravolini A, Williams DG, Thompson TL (2002) Carbon isotope discrimination and bundle sheath leakiness in three C4 subtypes grown under variable nitrogen, water and atmospheric CO2 supply. J Exp Bot 53:2261–2269
Furbank RT, Hatch MD (1987) Mechanism of C4 photosynthesis: the size and composition of the inorganic carbon pool in bundle sheath cells. Plant Physiol 85:958–964
Furbank RT, Jenkins CLD, Hatch MD (1989) CO2 concentrating mechanism of C4 photosynthesis – permeability of isolated bundle sheath cells to inorganic carbon. Plant Physiol 91:1364–1371
Furbank RT, Jenkins CLD, Hatch MD (1990) C4 photosynthesis: quantum requirement, C4 acid overcycling and Q-cycle involvement. Aust J Plant Physiol 17:553–558
Furbank RT, Hatch MD, Jenkins CLD (2000) C4 photosynthesis: mechanism and regulation. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Photosynthesis: physiology and metabolism. Kluwer Academic Publishers, The Netherlands, pp 435–457
Ghannoum O, Siebke K, von Caemmerer S, Conroy JP (1998) The photosynthesis of young Panicum C4 leaves is not C3-like. Plant Cell Env 21:1123–1131
Ghannoum O, Evans JR, Chow WS, Andrews TJ, Conroy JP, von Caemmerer S (2005) Faster Rubisco is the key to superior nitrogen use efficiency in NADP-ME relative to NAD-ME C4 grasses. Plant Physiol 137:638–650
Ghashghaie J, Badeck FW, Lanigan G, Nogués S, Tcherkez G, Deléens E, Cornic G, Griffiths H (2003) Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochem Rev 2:145–161
Gillon J, Yakir D (2001) Influence of carbonic anhydrase activity in terrestrial vegetation on the 18O content of atmospheric CO2. Science 291:2584–2587
Gutierrez M, Gracen VE, Edwards GE (1974) Biochemical and cytological relationships in C4 plants. Planta 119:279–300
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultra structure. Biochim Biophys Acta 895:81–106
Hatch MD, Agostino A, Jenkins CLD (1995) Measurement of the leakage of CO2 from bundle sheath cells of leaves during C4 photosynthesis. Plant Physiol 108:173–181
Hatch MD, Slack CD, Johnson HS (1967) Further studies on a new pathway of photosynthetic carbon dioxide fixation in sugarcane and its occurrence in other plant species. Biochem J 102:417–422
Hatch MD, Kagawa T, Craig S (1975) Subdivision of C4 pathway species based on different C4 acid decarboxylating systems and ultrastructural features. Aust J Plant Physiol 2:111–128
Hattersley PW, Browning AJ (1981) Occurence of suberized lamella in leaves of grasses of different photosyntetic types. I. In parenchymatous bundle sheaths and PCR (“kranz”) sheaths. Protoplasma 109:371–401
He D, Edwards GE (1996) Estimation of diffusive resistance of bundle sheath cells to CO2 from modelling of C4 photosynthesis. Photosynth Res 49:195–208
Henderson SA, von Caemmerer S, Farquhar GD (1992) Short term measurements of carbon isotope discrimination in several C4 species. Aust J Plant Physiol 19:263–285
Hobbie EA, Werner RA (2003) Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol 161:371–385
Jenkins CLD, Furbank RT, Hatch MD (1989) Inorganic carbon diffusion between C4 mesophyll and bundle sheath cells – direct bundle sheath CO2 assimilation in intact leaves in the presence of an inhibitor of the C4 pathway. Plant Physiol 91:1356–1363
Kanai R, Edwards G (1999) The biochemistry of C4 photosynthesis. In: Sage RF, Monson RK (eds) C4 plant biology. Academic Press, London, pp 49–87
Kiirats O, Lea PJ, Franceschi VR, Edwards GE (2002) Bundle sheath diffusive resistance to CO2 and effectiveness of C4 photosynthesis and refixation of photorespired CO2 in C4 cycle mutant and wild-type Amaranthus edulis. Plant Physiol 130:964–976
Kubásek J (2002) Diskriminace uhlíku 13C při fotosyntéze vyšších rostlin.(13C discrimination in higher-plants photosynthesis). Bachelor thesis in Czech, Faculty of Biology, University of South Bohemia, České Budějovice
Kubásek J (2004) C4 rostliny a stabilní izotopy. (C4 pants and stable isotopes). Diploma thesis in Czech, Faculty of Biology, University of South Bohemia, České Budějovice
Kubien DS, von Caemmerer S, Furbank RT, Sage F (2003) C4 photosynthesis at low temperature. A study using transgenic plants with reduced amounts of Rubisco. Plant Physiol 132:1577–1585
Lewis NG, Yamamoto E (1990) Lignin: occurrence, biogenesis and degradation. Ann Rev Plant Physiol Plant Mol Biol 41:455–496
Long SP (1999) Environmental responses. In: Sage RF, Monson RK (eds) C4 plants biology. Academic Press, London, pp 215–249
Ludwig M, von Caemmerer S, Price GD, Badger MR, Furbank RT (1998) Expression of tobacco carbonic anhydrase in the C4 dicot Flaveria bidentis leads to increase leakiness of the bundle sheath and a defective CO2 concentrating mechanism. Plant Physiol 117:1071–1081
Meinzer FC, Saliedra NZ (1997) Spatial patterns of carbon isotope discrimination and allocation of photosynthetic activity in sugarcane leaves. Aust J Plant Physiol 24:769–775
Mook WG, Bommerson JC, Staverman WH (1974) Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet Sci Lett 22:169–176
O’ Brien TP, Carr DJ (1970) A suberized layer in the cell walls of the bundle sheath of grasses. Aust J Biol Sci 23:275–287
Oberhuber W, Edwards GE (1993) Temperature dependence of the linkage of quantum yield of photosystem II to CO2 fixation in C4 and C3 plants. Plant Physiol 101:507–512
Peisker M, Henderson SA (1992) Carbon – terrestrial C4 plants. Plant Cell Env 15:987–1004
Pospíšilová J, Šantrůček J (1994) Stomatal patchiness. Biol Plant 36(4):481–510
Sage RF (2001) C4 Plants. In: Levin SA (ed) Encyclopedia of biodiversity. Academic Press, pp 575–598
Tazoe Y, Noguchi K, Terashima I (2006) Effects of growth light and nitrogen nutrition on the organisation of the photosynthetic apparatus in leaves of a C4 plant, Amaranthus cruentus. Plant Cell Env 29(4):691–700
Wang SY, Lin H (2006) Effect of plant growth temperature on membrane lipids in strawberry (Fragaria x ananassa Duch.). Sci Horticult 108(2006):35–42
Williams DG, Gempko V, Fravolini A, Leavitt SW, Wall GW, Kimball PJ, Pinter PJ Jr, LaMorte R, Ottman M (2001) Carbon isotope discrimination by Sorghum bicolor under CO2 enrichment and drought. New Phytol 150:285–293
Acknowledgments
We would like to thank R.F. Sage for providing us with seeds of Boerhavia coccinea and Mrs. Anička Ruprechtová for growing the plants. We are also grateful to reviewers (unfortunately anonymous) for many constructive comments and advices. The research was supported by Grant Agency of the Academy of Sciences of Czech Republic No. A601410505 and by MSM 6007665801 and AV0Z50510513 Grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubásek, J., Šetlík, J., Dwyer, S. et al. Light and growth temperature alter carbon isotope discrimination and estimated bundle sheath leakiness in C4 grasses and dicots. Photosynth Res 91, 47–58 (2007). https://doi.org/10.1007/s11120-007-9136-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-007-9136-6