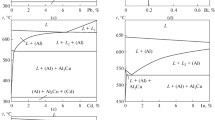
Data on the solidus surface of the Al–Cr–Fe system in the range 58–100 at.% Al and on the cast alloys examined with metallography, x-ray diffraction, and differential thermal and electron microprobe analyses are used to construct the liquidus surface of this system on the concentration triangle and study the processes that occur during crystallization of its alloys. This has permitted the construction of the Al–Cr–Fe melting diagram in the range 58–100 at.% Al. The liquidus surface includes primary crystallization fields of four ternary compounds, eight solid solutions based on binary phases, and a solid solution based on aluminum. There are fourteen four-phase invariant equilibria involving a liquid phase (three eutectic, two peritectic, and nine transition equilibria) and eight three-phase invariant equilibria involving a liquid phase.






Similar content being viewed by others
References
I. Kornikov, “Fe–Cr–Al alloys,” in: Iron Alloys [in Russian], Vol. 1, Izd. AN SSSR, Leningrad–Moscow (1945).
M. C. H. Taillandier, “Contribution to Al–Fe–Cr Alloys. Parts I and II,” Rev. Metal., 29, 315–325, 348–356 (1932).
L. F. Mondolfo, “Al–Cr–Fe,” in: Metallography of Aluminum Alloys, John Wiley and Sons Inc., New York (1943), pp. 70–71.
N. Saunders and V. G. Rivlin, “A critical review and thermodynamic calculations for the Al-rich portion of the Al–Cr–Fe phase diagram,” Z. Metallkd., 78, 795–801 (1987).
G. Ghosh, T. Velikanova, K. Korniyenko, and V. Sidorko, “Aluminum–chromium–iron,” in: Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology, W. Martienssen (ed.), New Series. Group IV: Physical Chemistry, G. Effenberg, S. Ilyenko (eds.), Ternary Alloy Systems, Phase Diagrams, Crystallographic and Thermodynamic Data, Vol. 11D1, Springer-Verlag, Berlin, Heidelberg (2007), pp. 30–66.
V. G. Khoruzha, K. E. Kornienko, and D. V. Pavlyuchkov, “The Al–Cr–Fe phase diagram. I. Phase equilibria at subsolidus temperatures over composition range 58–100 at.% Al,” Powder Metall. Met. Ceram., 50, No. 1–2, 83–97 (2011).
B. Grushko, E. Kowalska-Strzeciwilk, et al., “Investigation of the Al–Cr γ-range,” J. All. Compd., 402, 98–104 (2005).
B. Grushko, B. Przepiorzynski, and D. Pavlyuchkov, “On the constitution of the high-Al region of the Al–Cr alloy system,” J. All. Compd., 454, 214–220 (2008).
S. Balanetskyy, W. Kowalski, and B. Grushko, “Liquidus, solidus and reaction scheme of the Al-rich part of Al–Cr–Mn,” J. All. Compd., 474, 147–151 (2009).
Acknowledgement
The authors are grateful to D. O. Kapush for EMPA and to L. A. Duma for x-ray diffraction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 50, No. 3–4 (478), pp. 117–132, 2011.
Rights and permissions
About this article
Cite this article
Khoruzha, V.G., Kornienko, K.E., Pavlyuchkov, D.V. et al. The Al–Cr–Fe phase diagram. II. Liquidus surface and phase equilibria for crystallization of 58–100 at.% Al alloys. Powder Metall Met Ceram 50, 217 (2011). https://doi.org/10.1007/s11106-011-9321-1
Received:
Published:
DOI: https://doi.org/10.1007/s11106-011-9321-1