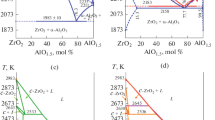
For better presentation of the Al2O3–HfO2–Y2O3 phase diagram over wide ranges of temperatures and concentrations, three vertical sections are plotted to show interactions in the ternary system. The Y2O3 bisector shows the Y2O3-rich region of the Al2O3–HfO2–Y2O3 phase diagram and explains the mechanism of X ⇆ H ⇆ A ⇆ B phase transformations for Y2O3 solid solutions. The 10 mol.% HfO2 (10H) isopleth shows the Al2O3–HfO2–Y2O3 structure in the region adjacent to the Al2O3–Y2O3 binary bounding system. The HfO2 bisector shows the structure of the HfO2-rich region and the mechanism of F ⇆ T ⇆ M phase transformations for HfO2 solid solutions.
Similar content being viewed by others
References
S. M. Lakiza, Ya. S. Tishchenko, V. P. Red’ko, et al., “Al2O3–HfO2–Y2O3 phase diagram. I. Isothermal sections at 1250 and 1650°C,” Powder Metall. Met. Ceram., 48, No. 3–4, 225–233 (2009).
S. M. Lakiza, Ya. S. Tishchenko, Z. O. Zaitseva, et al., “The Al2O3–HfO2–Y2O3 phase diagram. II. Liquidus surface,” Powder Metall. Met. Ceram., 48, No. 11–12, 693–699 (2009).
S. M. Lakiza, Ya. S. Tishchenko, L. M. Lopato, and A. O. Sus’, “The Al2O3–HfO2–Y2O3 phase diagram. III. Solidus surface and phase equilibria in crystallization of alloys,” Powder Metall. Met. Ceram., 49, No. 1–2, 71–78 (2010).
L. M. Lopato, A. V. Shevchenko, A. A. Frolov, and V. P. Red’ko, “Fusion and dispersion of oxide materials in a “cold” crucible and in furnaces with concentrated radiant heating,” Powder Metall. Met. Ceram., 44, No. 7–8, 335–340 (2005).
Y. Waku, S. Sakata, A. Mitani, and K. Shimizu, “A novel oxide composite reinforced with a ductile phase for very high temperature structural materials,” Mat. Res. Innovat., 2, No. 2, 94–100 (2001).
Y. Waku, S. Sakata, A. Mitani, et al., “Temperature dependence of flexural strength and microstructure of Al2O3/Y3Al5O12/ZrO2 ternary melt growth composites,” J. Mat. Sci., 37, No. 14, 2975–2982 (2002).
Y. Murayama, S. Hanada, J. H. Lee, et al., “High-temperature strength of directionally solidified Al2O3/YAG/ZrO2 eutectic composite,” Mat. Sci. Forum, 475–479, 1295–1300 (2005).
V. P. Red’ko, Physicochemical Study of M 4 Zr(Hf) 3 O 12 Compounds in ZrO 2 (HfO 2 )–Rare-Earth Oxide Systems [in Russian], Author’s Abstract of PhD Thesis, Kiev (1990), p. 20.
L. M. Lopato, A. V. Shevchenko, and G. I. Gerasimyuk, “System HfO2–Al2O3,” AN SSSR. Neorg. Mater., 12, No. 9, 1623–1626 (1976).
N. A. Toropov, I. A. Bondar, F. Ya. Galakhov, et al., “Phase equilibria in the yttria–alumina system,” Izv. AN SSSR. Ser. Khim., No. 7, 1158–1164 (1964).
I. A. Bondar, L. N. Koroleva, and E. T. Bezruk, “Physicochemical properties of yttrium aluminates and gallates,” Izv. AN SSSR. Neorg. Mater., 20, No. 2, 257–261 (1984).
B. Cocayne, “The uses and enigmas of the Al2O3–Y2O3 phase system,” J. Less-Common Met., 114, No. 1, 199–206 (1985).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 49, No. 3–4 (472), pp. 95–101, 2010.
Rights and permissions
About this article
Cite this article
Lakiza, S.M., Tishchenko, Y.S., Red’ko, V.P. et al. The Al2O3–HfO2–Y2O3 phase diagram. IV. Vertical sections. Powder Metall Met Ceram 49, 201–206 (2010). https://doi.org/10.1007/s11106-010-9222-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-010-9222-8