Abstract
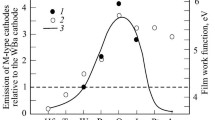
The effect of tungsten powder fineness and microstructure parameters of the tungsten skeleton on the emission of dispenser cathodes (DC) in the stages of degassing and high-temperature activation are studied. Quantitative analysis of the cathode surface microstructure is investigated. It is established that the work function of the DC after high-temperature activation does not depend upon the size of tungsten powder particles in the test range of fineness (average particle size 1.4–20 μm) and porosity of the tungsten skeleton (22 and 35%). The time for reaching the maximum DC activity increases with an increase in particle size and skeleton porosity. The highest emission uniformity is exhibited by cathodes with a uniform microstructure prepared from tungsten powder with an average size of 1 and 4 μm. It is shown that the DC emission capacity is connected with the marked three-dimensional structure of BaO-CaO at the cathode surface, and also monatomic films (Ba-O and Ba) and excess oxygen at the surface of the tungsten phase.
Similar content being viewed by others
References
G. A. Kudintseva, A. I. Mel’nikov, A. V. Morozov, and B. P. Nikonov, Thermoelectronic Cathodes [in Russian], Énergiya, Moscow; Leningrad (1966).
L. A. Vermenko, O. I. Get’man, and S. P Rakitin, “Effect of tungsten powder particle size on the structure and properties of dispenser cathodes (DC),” Élektron. Tekhnika, Ser. 6, Materialy, Issue 11, 25–32 (1980).
N. P. Brodnikovskii, L. A. Vermenko, O. I. Get’man, and S. P. Rakitin, “Structure and properties of barium-calcium aluminates (3-x) BaO · CaO · Al2O3,” Élektron. Tekhnika, Ser. 6, Materialy, Issue 4, 20–28 (1980).
O. I. Get’man, S. P. Rakitin, A. E. Korol’kov, et al., “Evolution of the microstructure of a dispenser cathode with a tungsten skeleton during operation,” Élektron. Tekhnika, Ser. 6, Materialy, Issue 5, 25–32 (1991).
V. F. Shnyukov, B. I. Mikhailovskii, A. E. Lushkin, et al., “Effect of vacuum heat treatment regime for dispenser cathodes on their physicochemical and emission properties,” Élektron. Tekhnika, Ser. 1, Élektronika SVCh, Issue 5, 24–28 (1991).
J. L. Cronin, “Modern dispenser cathodes,” IEEE Proc., 128, Part 1, 19–32 (1981).
D. H. Tomich, J. H. Mescher, P. N. Wittberg, et al., “Relative work function, surface composition and topography of “Pedigreed” impregnated tungsten dispenser cathodes,” Act. Surf. Sci., 24, 557–574 (1985).
A. S. Berkman and I. G. Mel’nikov, Porous Permeable Ceramics [in Russian], Stroizdat, Leningrad (1969).
V. F. Shnyukov, B. I. Mikhailovskii, A. E. Lushkin, et al., “Effect of decomposition for REM carbonates on the composition and emission capacity of the oxides obtained,” Izv. Akad. Nauk SSSR, Ser. Fiz., 52, No. 8, 1500–1503 (1988).
C. G. Pantano, “Electron beam damage in Auger electron spectroscopy,” Appl. Surf. Sci., 7, 115–141 (1981).
O. D. Protopopov, “Electron-stimulated effects in Auger spectroscopy,” Obzory po Élektronnoi Tekhnike. Ser. Élektronika SVCh, Central Research Inst. “É lektronika”, Moscow (1982).
J. A. Haas, A. Shih, and P. E. Thomas, “Electron and chemical surface studies in oxide cathodes,” Appl. Surf. Sci., 2, No. 2, 293–321 (1979).
E. N. Sickafus, M. A. Smith, J. S. Hammond, et al., “Surface phenomena of potential concern to longevity of dispenser cathodes,” Appl. Surf. Sci., 2, 213–231 (1979).
D. Jones, D. McNeely, and L. W. Swanson, “Surface and emission characterization of the of the impregnated dispenser cathode,” Appl. Surf. Sci., 2, 232–257(1979).
S. S. Volkov and A. B. Tolstoguzov, “Study of the composition of the surface of a compacted cathode by ion scattering,” Élektron. Tekhnika, Ser. 1, Élektronika SVCh, Issue 9(333), 25–27 (1981).
S. M. Solonin, “Calculation of the magnitude of the interphase surface of two-component powder mixtures,” Poroshk. Metall., Nos. 3–4, 37–41 (1994).
O. I. Get’man, S. P. Rakitin, V. V. Skorokhod, and A. E. Zuev, “Interconnection between powder fineness, pore dimensions, and sintered tungsten porous structure,” Poroshk. Metall., No. 12, 24–31 (1988).
V. F. Shnyukov, B. I. Mikhailovskii, A. E. Lushkin, et al., “Role of calcium in dispenser cathodes,” Izv. Akad. Nauk SSSR, Ser. Fiz., 5, No. 12, 2357–2361 (1991).
O. I. Get’man, A. E. Lushkin, V. V. Panichkina, et al., “Reason for the low emission capacity of dispenser cathodes,” Izv. RAN, Ser. Fiz., 58, No. 10, 76–79 (1994).
V. F. Shnyukov, B. I. Mikhailovskii, A. E. Lushkin, et al., “Study of the composition of dispenser cathodes by electron Auger-spectroscopy,” Élektron. Tekhnika, Ser. 1, Élektronika SVCh, Issue 8(392), 30–33 (1986).
C. R. K. Marrian, A. Shih, and G. A. Haas, “The characterization of the surfaces of tungsten-based dispenser cathodes,” Appl. Surf. Sci., 16, No. 1–2, 1–24 (1983).
A. P. Makarov, O. K. Kultashev, E. D. Kuranova, et al., “Mechanism of operation and ageing of an osmium treated dispenser cathode,” Radiotekhnika i Élektronika, 36, No. 11, 2196–2201 (1991).
A. N. Druzhinin, “Migration of barium over the surface of tungsten, molybdenum and rhenium coated with an absorbed gas film,” Radiotekhnika i Élektronika, No. 3, 496–504 (1965).
G. A. Haas, C. R. K. Marrian, and A. Shih, “Interpretation of AES data of impregnated cathodes,” Appl. Surf. Sci., 3–4, 430–446 (1985).
V. I. Kozlov, V. G. Vorozheikin, and Yu. I. Nabokov, “Some thermal emission properties of osmium treatred dispenser cathodes,” Élektron. Tekhnika, Ser. 1, Élektronika SVCh, Issue 11, 67–77 (1978).
B. A. Free and R. G. Gibson, “Dependence of surface coverage of pore geometry in dispenser cathodes,” Appl. Surf. Sci., 24, 358–371 (1985).
Author information
Authors and Affiliations
Additional information
__________
Translated from Poroshkovaya Metallurgiya, Nos. 11–12(446), pp. 97–108, November–December, 2005.
Rights and permissions
About this article
Cite this article
Get’man, O.I., Lushkin, A.E., Panichkina, V.V. et al. Effect of microstructure on the mechanism of emission for tungsten-barium dispenser cathodes. Powder Metall Met Ceram 44, 598–607 (2005). https://doi.org/10.1007/s11106-006-0031-z
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11106-006-0031-z