Abstract
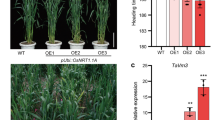
Protein phosphorylation–dephosphorylations are major signaling events induced by osmotic stress in plants. In this study, a wheat SNF1-related protein kinase 2 (SnRK2) gene, TaSRK2C1, was functionally characterized. The results from the sequence analysis showed that TaSRK2C1 contains conserved domains typified in SnRK2 protein kinases, including the ATP binding site, N-myristoylation site, protein kinase-activating signature, and transmembrane-spanning region. The transcripts of TaSRK2C1 in roots were induced by treatments of dehydration, high salinity, low temperature, and exogenous abscisic acid, which suggest its potential roles relative to osmotic stress signal transductions. The ectopic expression of TaSRK2C1 in tobacco significantly up-regulated the expression levels of three putative central regulators, namely, RD29a, DREB1A, and DREB2, which are involved in responding to osmotic stresses. Thus, higher levels of free proline and soluble carbohydrates in transgenic plants were detected, and conferred tolerance to high salinity, dehydration stress, and low temperature in plants. The overall results in this study indicate that TaSRK2C1 have important functions in plant response and adaptation to osmotic stresses via mediation of signal transductions initiated by distinct abiotic stresses. Manipulating TaSRK2C1 toward improving the osmotic-stress tolerance in crop plants is feasible.






Similar content being viewed by others
References
Boudsocq M, Droillard MJ, Barbier-Brygoo H, Lauriere C (2007) Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol Biol 63:491–503
Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103:1035–1040
Chandler PM, Robertson M (1994) Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 45:113–141
Chen JQ, Meng XP, Zhang Y, Xia M, Wang XP (2008) Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol Lett 30:2191–2198
Chen N, Yang Q, Su M, Pan L, Chi X, Chen M, He Y, Yang Z, Tong W, Wang M, Yu S (2012) Cloning of six ERF family transcription factor genes from peanut and analysis of their expression during abiotic stress. Plant Mol Biol Rep 30:1415–1425
Chung EH, da Cunha L, Wu AJ, Gao Z, Cherkis K, Afzal AJ, Mackey D, Dangl JL (2011) Specific threonine phosphorylation of a host target by two unrelated type III effectors activates a host innate immune receptor in plants. Cell Host Microbe 9:125–136
Claussen W (2005) Proline as a measure of stress in tomato plants. Plant Sci 168:241–248
Diedhiou CJ, Popova OV, Dietz KJ, Golldack D (2008) The SNF1-type serine-threonine protein kinase SAPK4 regulates stress-responsive gene expression in rice. BMC Plant Biol 8:49
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33:751–763
Farras R, Ferrando A, Jasik J, Kleinow T, Okresz L, Tiburcio A, Salchert K, del Pozo C, Schell J, Koncz C (2001) SKP1-SnRK protein kinase interactions mediate proteasomal binding of a plant SCF ubiquitin ligase. EMBO J 20:2742–2756
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signalling networks. Curr Opin Plant Biol 9:436–442
Gilmour SJ, Thomashow MF (1991) Cold acclimation and cold-regulated gene expression in ABA mutants of Arabidopsis thaliana. Plant Mol Biol 17:1233–1240
Gosti F, Bertauche N, Vartanian N, Giraudat J (1995) Abscisic acid-dependent and independent regulation of gene expression by progressive drought in Arabidopsis thaliana. Mol Gen Genet 246:10–18
Guo L, Zhao Y, Zhang S, Zhang H, Xiao K (2009) Improvement of organic phosphate acquisition in transgenic tobacco plants by overexpression of a soybean phytase gene Sphy1. Front Agric China 3:259–265
Halford NG, Hey SJ (2009) Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem J 419:247–259
Halfter U, Ishitani M, Zhu JK (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein, SOS3. PNAS 97:3535–3540
He L, Gao C, Wang Y, Wu Y, Zhi L (2012) A basic Helix–Loop–Helix gene from poplar is regulated by a basic leucine-zipper protein and is involved in the ABA-dependent signaling pathway. Plant Mol Biol Rep. doi:10.1007/s11105-012-0507-6
Hoyos ME, Zhang S (2000) Calcium-independent activation of salicylic acid-induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol 122:1355–1363
Hrabak EM, Chan CW, Gribskov M, Harper JF, Choi JH, Halford N, Kudla J, Luan S, Nimmo HG, Sussman MR, Thomas M, Walker-Simmons K, Zhu JK, Harmon AC (2003) The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol 132:666–680
Huai J, Wang M, He J, Zheng J, Dong Z, Lv H, Zhao J, Wang G (2008) Cloning and characterization of the SnRK2 gene family from Zea mays. Plant Cell Rep 27:1861–1868
Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24:655–665
Ishitani M, Xiong L, Stevenson B, Zhu JK (1997) Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9:1935–1949
Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T (2004) Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 16:1163–1177
Kumar SG, Reddy AM, Sudhakar C (2003) NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Sci 165:1245–1251
Li J, Assmann SM (1996) An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell 8:2359–2368
Li J, Wang XQ, Watson MB, Assmann SM (2000) Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287:300–303
Li F, Lei H, Zhao X, Tian R, Li T (2012) Characterization of three sorbitol transporter genes in micropropagated apple plants grown under drought stress. Plant Mol Biol Rep 30:123–130
Liu J, Ishitani M, Halfter U, Kim CS, Zhu JK (2000) The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. PNAS 97:3730–3734
Liu J, Elmore JM, Lin ZJ, Coaker G (2011) A receptor-like cytoplasmic kinase phosphorylates the host target RIN4, leading to the activation of a plant innate immune receptor. Cell Host Microbe 9:137–146
Mao XG, Zhang HY, Tian SJ, Chang XP, Xie HM, Jing RL (2010) TaSnRK2.4, a SNF1-type serine-threonine protein kinase of wheat (Triticum aestivum L.) confers enhanced multi-stress tolerance in Arabidopsis. J Exp Bot 61:683–696
Mikolajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G (2000) Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12:165–178
Munnik T, Ligterink W, Meskiene I, Calderini O, Beyerly J, Musgrave A, Hirt H (1999) Distinct osmo-sensing protein kinase pathways are involved in signalling moderate and severe hyper-osmotic stress. Plant J 20:381–388
Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Cell 14:3089–3099
Nordin K, Heino P, Palva ET (1991) Separate signal pathways regulate the expression of a low-temperature-induced gene in Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 17:1233–1240
Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, Yang GD, Gao Z, Zheng CC (2007) Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol 176:70–81
Shen Q, Ho THD (1995) Functional dissection of an abscisic acid (ABA)-inducible gene reveals two independent ABAresponsive complexes each containing a G-box and a novel cis-acting element. Plant Cell 7:295–307
Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2:503–512
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CEF 1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. PNAS 94:1035–1040
Umezawa T, Yoshida R, Maruyama K, Yamaguchi-Shinozaki K, Hinozaki K (2004) SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana. PNAS 101:17306–17311
Wen JQ, Oono K, Imai R (2002) Two novel mitogen-activated protein signaling components, OsMEK1 and OsMAP1, are involved in a moderate low-temperature signaling pathway in rice. Plant Physiol 129:1880–1891
Xiang Y, Huang Y, Xiong L (2007) Characterization of stress responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol 144:1416–1428
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25:131–139
Yamaguchi-Shinozaki K, Shinozaki K (1994) A nove1 cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43:1473–1483
Yu S, Zhang F, Yu Y, Zhang D, Zhao X, Wang W (2012) Transcriptome profiling of dehydration stress in the Chinese cabbage (Brassica rapa L. ssp. pekinensis) by tag sequencing. Plant Mol Biol Rep 30:17–28
Zeevaart JAD, Creelman RA (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39:439–473
Zhang H, Guo C, Li C, Xiao K (2008) Cloning, characterization and expression analysis of two superoxide dismutase (SOD) genes in wheat (Triticum aestivum L.). Front Agric China 2:141–149
Zhou G, Yang L-T, Li Y-R, Zou C-L, Huang L-P, Qiu L-H, Huang X, Srivastava MK (2012) Proteomic analysis of osmotic stress-responsive proteins in sugarcane leaves. Plant Mol Biol Rep 30:349–359
Zhu JK, Hasegawa PM, Bressan RA (1997) Molecular aspects of osmotic stress in plants. Crit Rev Plant Sci 16:253–277
Acknowledgments
This work was supported by the National Transgenic Major Program (No. 2011ZX08008), Natural Science Foundation of Hebei (No. C2010000752 and No. C2010000720) and the Key Crop Growth Regulation Laboratory of Hebei Province. The authors thank two anonymous reviewers and the editorial board staff whose detailed and valuable comments helped improve the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
X. Du and X. Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Du, X., Zhao, X., Li, X. et al. Overexpression of TaSRK2C1, a Wheat SNF1-Related Protein Kinase 2 Gene, Increases Tolerance to Dehydration, Salt, and Low Temperature in Transgenic Tobacco. Plant Mol Biol Rep 31, 810–821 (2013). https://doi.org/10.1007/s11105-012-0548-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-012-0548-x