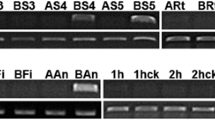
Abstract
A polygalacturonase gene, designated BcMF17 (Brassica campestris Male Fertility 17), has been cloned from Brassica campastris. This gene, encoding polygalacturonase (PG), consists of a 1,194 bp open reading frame along with four exons and three introns, and exhibits 79% similarities to BcMF9 (Huang et al. 2009). Detailed multiple amino acid sequence alignment and phylogenetic tree analysis indicate that BcMF17 belongs to a pollen clade and may be involved in pollen wall formation. Multiple alignments against homologous sequences from the genus Brassica indicate that BcMF17 is highly conserved in this genus. Quantitative real-time PCR results indicate that transcripts of BcMF17 are preferentially expressed in reproductive tissues, especially abundant in male floral tissues, but are expressed at low level in vegetative tissues. BcMF17 transcripts in male floral tissues persist from the anther primordia stage till to the mature pollen stage. In situ hybridized signals of BcMF17 are highly detected during anther development. During anther development, expression patterns of BcMF17 are very similar to those of BcMF9; however, unlike BcMF9, BcMF17 is continuously expressed during this period, and its transcripts are detected in the whole plant during its life cycle.





Similar content being viewed by others
Abbreviations
- BcMF17 :
-
Brassica campestris Male Fertility 17
- BcMF9 :
-
Brassica campestris Male Fertility 9
- qRT-PCR:
-
quantitative Real-time polymerase chain reaction
- PG:
-
Polygalacturonase
- cDNA-AFLP:
-
cDNA–amplified fragment length polymorphism
References
Alam MM, Sharmin S, Nabi Z, Mondal SI, Islam MS, Bin Nayeem S, Shoyaib M, Khan H (2010) A putative leucine-rich repeat receptor-like kinase of jute involved in stress response. Plant Mol Biol Rep 28:394–402
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: signalp 3.0. J Mol Biol 340:783–795
Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47:311–340
Chen W, Baldwin TC (2007) An improved method for the fixation, embedding and immunofluorescence labeling of resin-embedded plant tissue. Plant Mol Biol Rep 25:27–35
Dubald M, Barakate A, Mandaron P, Mache R (1993) The ubiquitous presence of exopolygalacturonase in maize suggests a fundamental cellular function for this enzyme. Plant J 4:781–791
Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300:1005–1016
Hadfield KA, Bennett AB (1998) Polygalacturonases: many genes in search of a function. Plant Physiology 117:337–343
Hadfield KA, Rose JKC, Yaver DS, Berka RM, Bennett AB (1998) Polygalacturonase gene expression in ripe melon fruit supports a role for polygalacturonase in ripening-associated pectin disassembly. Plant Physiology 117:363–373
Hiller K, Grote A, Scheer M, Munch R, Jahn D (2004) PrediSi: prediction of signal peptides and their cleavage positions. Nucleic Acids Res 32:W375–W379
Holmes-Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S (2005) Proteome mapping of mature pollen of Arabidopsis thaliana. Proteomics 5:4864–4884
Honys D, Twell D (2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 132:640–652
Huang L, Cao JS, Ye WZ, Liu TT, Jiang LX, Ye YQ (2008a) Transcriptional differences between the male-sterile mutant bcms and wild-type Brassica campestris ssp. chinensis reveal genes related to pollen development. Plant Biol 10:342–355
Huang L, Cao JS, Zhang AH, Ye YQ (2008b) Characterization of a putative pollen-specific arabinogalactan protein gene, BcMF8, from Brassica campestris ssp. Chinensis. Mol Biol Rep 35:631–639
Huang L, Ye YQ, Zhang YC, Zhang AH, Liu TT, Cao JS (2009) BcMF9, a novel polygalacturonase gene, is required for both Brassica campestris intine and exine formation. Ann Bot 104:1339–1351
Huang L, Zhao XF, Liu TT, Dong H, Cao JS (2010) Developmental characteristics of floral organs and pollen of Chinese cabbage (Brassica campestris L. ssp chinensis). Plant Syst Evol 286:103–115
Hulzink RJM, Weerdesteyn H, Croes AF, Gerats T, van Herpen MMA, van Helden J (2003) In silico identification of putative regulatory sequence elements in the 5 '-untranslated region of genes that are expressed during male gametogenesis. Plant Physiol 132:75–83
Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN (2005) MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 17:2981–2992
Kato H, Xie GS, Sato Y, Imai R (2010) Isolation of Anther-specific gene promoters suitable for transgene expression in rice. Plant Mol Biol Rep 28:381–387
Kim J, Shiu SH, Thoma S, Li WH, Patterson SE (2006) Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biol 7:R87
Kohli DK, Bachhawat AK (2003) CLOURE: Clustal Output Reformatter, a program for reformatting ClustalX/ClustalW outputs for SNP analysis and molecular systematics. Nucleic Acids Res 31:3501–3502
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 5:150–163
Lau JM, Cooper NG, Robinson DL, Korban SS (2009) Sequence and in silico characterization of the tomato polygalacturonase (PG) promoter and terminator regions. Plant Mol Biol Rep 27:250–256
Lee BH, Henderson DA, Zhu JK (2005) The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 17:3155–3175
Liu AH, Wang JB (2006) Genomic evolution of Brassica allopolyploids revealed by ISSR marker. Genet Resour Crop Ev 53:603–611
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Louw C, Young PR, van Rensburg P, Divol B (2010) Regulation of endo-polygalacturonase activity in Saccharomyces cerevisiae. FEMS Yeast Res 10:44–57
Malik MR, Wang F, Dirpaul JM, Zhou N, Hammerlindl J, Keller W, Abrams SR, Ferrie AMR, Krochko JE (2008) Isolation of an embryogenic line from non-embryogenic Brassica napus cv. Westar through microspore embryogenesis. J Exp Bot 59:2857–2873
Mascarenhas JP (1990) Gene activity during pollen development. Annu Rev Plant Phys 41:317–338
Meng CM, Zhang TZ, Guo WZ (2009) Molecular cloning and characterization of a novel gossypium hirsutum L. bHLH gene in response to ABA and drought stresses. Plant Mol Biol Rep 27:381–387
Nielsen H, Engelbrecht J, Brunak S, von Heijne G (1997) A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int J Neural Syst 8:581–599
Park KC, Kwon SJ, Kim PH, Bureau T, Kim NS (2008) Gene structure dynamics and divergence of the polygalacturonase gene family of plants and fungus. Genome 51:30–40
Parkin IAP, Gulden SM, Sharpe AG, Lukens L, Trick M, Osborn TC, Lydiate DJ (2005) Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana. Genetics 171:765–781
Punwani JA, Rabiger DS, Drews GN (2007) MYB98 positively regulates a battery of synergid-expressed genes encoding filiform apparatus localized proteins. Plant Cell 19:2557–2568
Qi JN, Yu SC, Zhang FL, Shen XQ, Zhao XY, Yu YJ, Zhang DS (2010) Reference gene selection for real-time quantitative polymerase chain reaction of mRNA transcript levels in chinese cabbage (Brassica rapa L. ssp pekinensis). Plant Mol Biol Rep 28:597–604
Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5:e1000621
Qiu X, Erickson L (1996) A pollen-specific polygalacturonase-like cDNA from alfalfa. Sex Plant Reprod 9:123–124
Rhee SY, Osborne E, Poindexter PD, Somerville CR (2003) Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiol 133:1170–1180
Robert LS, Allard S, Gerster JL, Cass L, Simmonds J (1993) Isolation and characterization of a polygalacturonase gene highly expressed in Brassica napus pollen. Plant Mol Biol 23:1273–1278
Sitrit Y, Hadfield KA, Bennett AB, Bradford KJ, Downie AB (1999) Expression of a polygalacturonase associated with tomato seed germination. Plant Physiol 121:419–428
Taylor JE, Webb STJ, Coupe SA, Tucker GA, Roberts JA (1993) Changes in polygalacturonase activity and solubility of poluronides during ethylene-stimulated leaf abscission in Sambucus-Nigra. J Exp Bot 44:93–98
Verlent I, Van Loey A, Smout C, Duvetter T, Hendrickx ME (2004) Purified tomato polygalacturonase activity during thermal and high-pressure treatment. Biotechnol Bioeng 86:63–71
Villarreal NM, Bustamante CA, Civello PM, Martinez GA (2010) Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J Sci Food Agr 90:683–689
Wang YQ, Ye WZ, Cao JS, Yu XL, Xiang X, Lu G (2005) Cloning and characterization of the microspore development-related gene BcMF2 in Chinese cabbage Pak-Choi (Brassica campestris L. ssp. chinensis Makino). J Integr Plant Biol 47:9
Wang J, Tian N, Huang X, Chen LY, Schlappi M, Xu ZQ (2009) The tall fescue turf grass class I Chitinase gene FaChit1 is activated by fungal elicitors, dehydration, ethylene, and mechanical wounding. Plant Mol Biol Rep 27:305–314
Yoshida O, Nakagawa H, Ogura N, Sato T (1984) Effect of heat-treatment on the development of polygalacturonase activity in tomato fruit during ripening. Plant Cell Physiol 25:505–509
Zhang LL, Chen KS, Xu CJ (2008a) Identification and characterization of transcripts differentially expressed in peel and juice vesicles of immature and ripe orange (Citrus sinensis) fruit. Plant Mol Biol Rep 26:121–132
Zhang Q, Huang L, Liu TT, Yu XL, Cao JS (2008b) Functional analysis of a pollen-expressed polygalacturonase gene BcMF6 in Chinese cabbage (Brassica campestris L. ssp chinensis Makino). Plant Cell Rep 27:1207–1215
Zhu Y, Dun XL, Zhou ZF, Xia SQ, Yi B, Wen J, Shen JX, Ma CZ, Tu JX, Fu TD (2010) A separation defect of tapetum cells and microspore mother cells results in male sterility in Brassica napus: the role of abscisic acid in early anther development. Plant Mol Biol 72:111–123
Acknowledgments
This work was supported by the Chinese Natural Science Foundation (No. 31071805).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 60 kb)
Rights and permissions
About this article
Cite this article
Zhang, A., Chen, Q., Huang, L. et al. Cloning and Expression of an Anther-Abundant Polygalacturonase Gene BcMF17 from Brassica Campestris ssp. Chinensis . Plant Mol Biol Rep 29, 943–951 (2011). https://doi.org/10.1007/s11105-011-0298-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-011-0298-1