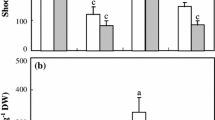
Abstract
Background and aims
Populus bolleana Lauche. (P. bolleana) and Populus euphratica Oliv. (P. euphratica) separately survive in mild and moderate alkaline soil conditions. The aim of this study was to explore the underlying mechanism for the different alkaline tolerance in the two poplar species.
Methods
Young saplings of two poplar species were grown in moderate alkaline soil, and the young and old leaves of the two poplars were separately analyzed by ion concentration, allocation and distribution, transcript variation of different genes involved in ion transport and nitrogen assimilation, nitrogen metabolism, organic acid, leaf pigments, and redox responses.
Results
Excess Na+ under alkali stress was mainly allocated to old leaves in P. bolleana. However, excess Na+ was allocated to both young and old leaves in P. euphratica, and was balanced by enhanced levels of Mg2+, Ca2+, and SO42−, with no change in oxidative parameter. The reduction of nitrate nitrogen occurred under alkali stress in both species; P. euphratica acclimated to alkali stress by more flexible regulation of N metabolism and nitrate absorption than P. bolleana.
Conclusions
Our results strongly indicated different alkali tolerance mechanisms in P. bolleana and P. euphratica. P. bolleana protects young tissues via profound accumulation of Na+ and confining damage effects into the old leaves under alkali stress, while P. euphratica can effectively compartmentalize excess Na+, keep its ion balance, and adjust nitrogen transport and metabolism in both young and old leaves to avoid alkali damage.







Similar content being viewed by others
Abbreviations
- NHX:
-
Na+/H+ antiporter
- SOS1:
-
Salt overly sensitive
- AMT:
-
Ammonium transporter
- NRT:
-
Nitrate transporter
- NR:
-
Nitrate reductase
- NiR:
-
Nitrite reductase
- AS:
-
Asparagine synthetase
- GS:
-
Glutamine synthetase;1
- AspAT:
-
Aspartate aminotransferase
- GDH:
-
Glutamate dehydrogenase
- NADH:
-
Nicotinamide adenine dinucleotide
- NADH-GOGAT:
-
NADH-dependent glutamine-2-oxoglutarate aminotransferase
- SOD:
-
Superoxide dismutase
- Fd-GOGAT:
-
Ferredoxin-dependent glutamate synthase
- HKT:
-
High-affinity potassium transporter
- HAK:
-
High-affinity potassium transporter
- MDA:
-
Malondialdehyde
- CAT:
-
catalase
- POD:
-
Peroxidase
- OH− :
-
Hydroxide anion
- OAs:
-
Organic acids
- HPLC:
-
High performance liquid chromatography
- qRT-PCR:
-
Qualitatively real-time PCR
- ROS:
-
Reactive oxygen species
- TBARS:
-
Thiobarbituric acid reactive substance
- PE:
-
Spectrophotometer
- LSD:
-
Least significance difference
- TCA:
-
Tricarboxylic acid cycle
References
Aleman F, Nieves-Cordones M, Martínez V, Rubio F (2009) Potassium/sodium steady-state homeostasis in Thellungiella halophila and Arabidopsis thaliana under long-term salinity conditions. Plant Sci 176(6):768–774
Assaad HI, Hou Y, Zhou L, Carroll RJ, Wu G (2015) Rapid publication-ready MS-Word tables for two-way ANOVA. Springerplus 4(1):33
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161(2):559–566
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta Biomembr 1465(1):140–151
Brunner AM, Busov VB, Strauss SH (2004) Poplar genome sequence: functional genomics in an ecologically dominant plant species. Trends Plant Sci 9(1):49–56
Cazzonelli CI (2011) Carotenoids in nature: insights from plants and beyond. Funct Plant Biol 38(11):833–847
Chen S, Li J, Yin W, Wang S, Fritz E, Polle A, Hüttermann A (2002) Tissue and cellular K+, Ca2+ and Mg2+ of poplar under saline conditions. J Beijing For Univ 24(5):84–88
Chen W, Cui P, Sun H, Guo W, Yang C, Jin H, Fang B, Shi D (2009a) Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.) Ind Crop Prod 30(3):351–358
Chen F, Liu X, Chen L (2009b) Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity. Food Chem 114(2):657–664
Couturier J, Montanini B, Martin F, Brun A, Blaudez D, Chalot M (2007) The expanded family of ammonium transporters in the perennial poplar plant. New Phytol 174(1):137–150
Crosby N (1968) Determination of ammonia by the Nessler method in waters containing hydrazine. Analyst 93(1107):406–408
De la Torre WR, Burkey KO (1990) Acclimation of barley to changes in light intensity: chlorophyll organization. Photosynth Res 24(2):117–125
Ding M, Hou P, Shen X, Wang M, Deng S, Sun J, Xiao F, Wang R, Zhou X, Lu C, Zhang D, Zheng X, Hu Z, Chen S (2010) Salt-induced expression of genes related to Na+/K+ and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant Mol Biol 73(3):251–269
Dluzniewska P, Gessler A, Dietrich H, Schnitzler JP, Teuber M, Rennenberg H (2007) Nitrogen uptake and metabolism in Populus× canescens as affected by salinity. New Phytol 173(2):279–293
Drechsler N, Zheng Y, Bohner A, Nobmann B, von Wiren N, Kunze R, Rausch C (2015) Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiol 169(4):2832–2847
Dvořák J, Noaman M, Goyal S, Gorham J (1994) Enhancement of the salt tolerance of Triticum turgidum L. by the Kna1 locus transferred from the Triticum aestivum L. chromosome 4D by homoeologous recombination. TAG Theor Appl Genet 87(7):872–877
Ehlting B, Dluzniewska P, Dietrich H, Selle A, Teuber M, Hansch R, Nehls U, Polle A, Schnitzler JP, Rennenberg H, Gessler A (2007) Interaction of nitrogen nutrition and salinity in Grey poplar (Populus tremula× alba). Plant Cell Environ 30(7):796–811
Fan SC, Lin CS, Hsu PK, Lin SH, Tsay YF (2009) The Arabidopsis nitrate transporter NRT1. 7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell 21(9):2750–2761
Flowers T, Hajibagheri M, Clipson N (1986) Halophytes. Q Rev Biol 61(3):313–337
Gorham J, Jones RW, Bristol A (1990) Partial characterization of the trait for enhanced K(+)-(Na+) discrimination in the D genome of wheat. Planta 180(4):590–597
Hajlaoui H, El Ayeb N, Garrec JP, Denden M (2010) Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind Crop Prod 31(1):122–130
Havaux M, Tardy F (1999) Loss of chlorophyll with limited reduction of photosynthesis as an adaptive response of Syrian barley landraces to high-light and heat stress. Funct Plant Biol 26(6):569–578
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agric Exp Stat 347:1–32
Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A (2001) Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J 27(2):129–138
Husted JA, Cook RJ, Farewell VT, Gladman DD (2000) Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol 53(5):459–468
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57(2):315–319
Kinnersley AM, Turano FJ (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19(6):479–509
Li C, Fang B, Yang C, Shi D, Wang D (2009) Effects of various salt–alkaline mixed stresses on the state of mineral elements in nutrient solutions and the growth of alkali resistant halophyte Chloris virgata. J Plant Nutr 32(7):1137–1147
Li J, Fu Y, Pike SM, Bao J, Tian W, Zhang Y, Chen C, Zhang Y, Li H, Huang J (2010) The Arabidopsis nitrate transporter NRT1. 8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 22(5):1633–1646
Lin S, Kuo H, Canivenc G, Lin C, Lepetit M, Hsu PK, Tillard P, Lin HL, Wang YY, Tsai CB (2008) Mutation of the Arabidopsis NRT1. 5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20(9):2514–2528
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Meng S, Peng J, He Y, Zhang G, Yi H, Fu Y, Gong J (2016) Arabidopsis NRT1.5 mediates the suppression of nitrate starvation-induced leaf senescence by modulating foliar potassium level. Mol Plant 9(3):461–470
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25(2):239–250
Nieves-Cordones M, Alemán F, Martínez V, Rubio F (2010) The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol Plant 3(2):326–333
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60(3):324–349
Rausch T, Kirsch M, Löw R, Lehr A, Viereck R, Zhigang A (1996) Salt stress responses of higher plants: the role of proton pumps and Na+/H+-antiporters. J Plant Physiol 148(3–4):425–433
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133(4):651–669
Storey R, Walker RR (1999) Citrus and salinity. Sci Hortic 78:39–81
Venema K, Quintero FJ, Pardo JM, Donaire JP (2002) The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J Biol Chem 277(4):2413–2418
Vorob’eva LA, Pankova EI (2008) Saline-alkali soils of Russia. Eurasian Soil Sci 41(5):457–470
Wang Y, Tsay YF (2011) Arabidopsis nitrate transporter NRT1. 9 is important in phloem nitrate transport. Plant Cell Online 23(5):1945–1957
Wang Y, Ma H, Liu G, Xu C, Zhang D, Ban Q (2008) Analysis of gene expression profile of Limonium bicolor under NaHCO3 stress using cDNA microarray. Plant Mol Biol Report 26(3):241–254
Wang H, Ahan J, Wu Z, Shi D, Liu B, Yang C (2012a) Alteration of nitrogen metabolism in rice variety ‘Nipponbare’ induced by alkali stress. Plant Soil 355(1–2):131–147
Wang H, Wu Z, Han J, Zheng W, Yang C (2012b) Comparison of ion balance and nitrogen metabolism in old and young leaves of alkali-stressed rice plants. PLoS One 7(5):e37817
Wang YY, Hsu PK, Tsay YF (2012c) Uptake, allocation and signaling of nitrate. Trends Plant Sci 17(8):458–467
Xie H (1999) Determination of nitrogen content in nitrate by salicylic acid colorimetry in water. Guizhou Agric Sci 27(3):40–41
Yang C, Chong J, Li C, Kim C, Shi D, Wang D (2007) Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294(1–2):263–276
Yang C, Jianaer A, Li C, Shi D, Wang D (2008a) Comparison of the effects of salt-stress and alkali-stress on photosynthesis and energy storage of an alkali-resistant halophyte Chloris virgata. Photosynthetica 46(2):273–278
Yang C, Shi D, Wang D (2008b) Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.) Plant Growth Regul 56(2):179–190
Yang C, Guo W, Shi D (2010) Physiological roles of organic acids in alkali-tolerance of the alkali-tolerant halophyte. Agron J 102(4):1081
Zhang X, Xiao X, Sun Y (2015) Different physiological response of different maturity of leaves of Populus bolleana to alkali stress. For Sci 51(12):9–16
Zou Q (2000) Plant physiology experiment instruction. China agriculture press, Beijing
Funding
This research was supported by the Youth Foundation of Science and Technology in Sichuan, China, (No. 2014JQ0016), Natural Science Foundation of China (31770644 and 31270660), Project of Innovation research team in the Sichuan Education Administration (No. 13TD0023), and the Longshan Talent Program of Southwestern University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Honghua He
Electronic supplementary material
Fig. S1
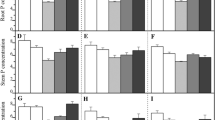
Effects of alkali stress on the expression of genes involved in Na+ absorption and metabolism in young and old leaves of two poplars. White bars indicate young leaves of control (CK-Young), light gray bars indicate old leaves of control (CK-Old), gray bars indicate young leaves under alkali treatment (A-Young), and black bars indicate old leaves under alkali treatment (A-Old). Columns represent values which are means (± SE) of four biological replicates. Statistically significant between organs at same stress condition, different letters on the bars indicate significant difference. P values of the ANOVAs of species, control, alkali treatment, and their interaction are indicated. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant (GIF 36 kb)
Fig. S2
Effects of alkali stress on the expression of HAK gene family in young and old leaves of two poplars. White bars indicate young leaves of control (CK-Young), light gray bars indicate old leaves of control (CK-Old), gray bars indicate young leaves under alkali treatment (A-Young), and black bars indicate old leaves under alkali treatment (A-Old). Columns represent values which are means (± SE) of four biological replicates. Statistically significant between organs at same stress condition, different letters on the bars indicate significant difference. P values of the ANOVAs of species, control, alkali treatment, and their interaction are indicated. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant (GIF 64 kb)
Fig. S3
Effects of alkali treatment on the expression of NRT gene family in young and old leaves of two poplars. White bars indicate young leaves of control (CK-Young), light gray bars indicate old leaves of control (CK-Old), gray bars indicate young leaves under alkali treatment (A-Young), and black bars indicate old leaves under alkali treatment (A-Old). Columns represent values which are means (± SE) of four biological replicates. Statistically significant between organs at same stress condition, different letters on the bars indicate significant difference. P values of the ANOVAs of species, control, alkali treatment, and their interaction are indicated. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant (GIF 28 kb)
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Sun, Y., Ou, Y., Gao, Y. et al. Different tolerance mechanism to alkaline stresses between Populus bolleana and its desert relative Populus euphratica. Plant Soil 426, 349–363 (2018). https://doi.org/10.1007/s11104-018-3632-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3632-7