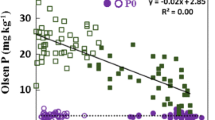
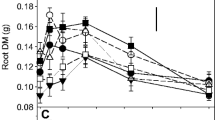
Abstract
Background and aims
Each genotype within species has a particular combination of root morphological and/or physiological traits to adapt to phosphorus-limited environments, which can lead to its unique plant fitness and competitive ability. Yet, how the various phosphorus environments affect the competition between genotypes remains obscure.
Methods
Two maize (Zea mays L.) genotypes (XY335 and HMY, bred in nutrient-rich and nutrient-poor environments, respectively) were grown in monoculture and mixture in phosphorus-limited soil with homogeneous or heterogeneous supply patterns and inorganic (Pinorg) or organic phosphorus (Porg) forms.
Results
In homogeneous Pinorg and Porg environments, XY335 had higher root length and surface area, but lower mycorrhizal colonization and the acid phosphatase and phytase activities in the rhizosphere, than HMY. In heterogeneous phosphorus environments, XY335 had higher root proliferation than HMY. The root trait divergence influenced the competition in mixture: XY335 had a competitive advantage compared to HMY under heterogeneous phosphorus conditions, whereas HMY exhibited a stronger competitive ability in homogeneous phosphorus treatments; these reverse trends were more significant in the Porg than Pinorg treatments.
Conclusions
The results suggested the importance of root physiological traits in homogeneous phosphorus-limited soil environments, whereas P acquisition strategy based on root morphological traits favours heterogeneous phosphorus supply.





Similar content being viewed by others
References
Abbott JM, Stachowicz JJ (2016) The relative importance of trait vs. genetic differentiation for the outcome of interactions among plant genotypes. Ecology 97:84–94
Adler PB, Fajardo A, Kleinhesselink AR, Kraft NJ (2013) Trait-based tests of coexistence mechanisms. Ecol Lett 16:1294–1306
Barot S, Bornhofen S, Boudsocq S, Raynaud X, Loeuille N (2016) Evolution of nutrient acquisition: when space matters. Funct Ecol 30:283–294
Bates TR, Lynch JP (2001) Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 236:243–250
Bennett JA, Riibak K, Tamme R, Lewis RJ, Pärtel M (2016) The reciprocal relationship between competition and intraspecific trait variation. J Ecol 104:1410–1420
Cahill JF (2013) Plant competition: can understanding trait-behavior linkages offer a new perspective on very old questions. Nova Acta Leopold 114:115–125
Ceulemans T, Bode S, Bollyn J, Harpole W, Coorevits C, Peeters G, Acker KV, Smolders E, Boeckx P, Honnay O (2017) Phosphorus resource partitioning shapes phosphorus acquisition and plant species abundance in grasslands. Nat Plant 3:16224
Chamberlain SA, Bronstein JL, Rudgers JA (2014) How context dependent are species interactions? Ecol Lett 17:881–890
Chen W, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM (2016) Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc Natl Acad Sci U S A 113:8741–8746
Chen W, Koide RT, Eissenstat DM (2017) Root morphology and mycorrhizal type strongly influence root production in nutrient hot spots of mixed forests. J Ecol 106:148–156. https://doi.org/10.1111/1365-2745.12800
Chesson P (2000) Mechanisms of maintenance of species diversity. Ann Rev Ecol Syst 31:343–366
Chu Q, Wang X, Yang Y, Chen F, Zhang F, Feng G (2013) Mycorrhizal responsiveness of maize (Zea mays L.) genotypes as related to releasing date and available P content in soil. Mycorrhiza 23:497–505
Craine JM, Dybzinski R (2013) Mechanisms of plant competition for nutrients, water and light. Funct Ecol 27:833–840
Dalal RC (1997) Long-term phosphorus trends in Vertisols under continuous cereal cropping. Aust J Soil Res 35:327–339
Day KJ, John EA, Hutchings MJ (2003) The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina. Funct Ecol 17:454–463
de Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ (2009) A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant Cell Environ 32:704–712
Forde BG (2002) Local and long-range signaling pathways regulating plant responses to nitrate. Ann rev. Plant Biol 53:203–224
Fort F, Cruz P, Jouany C, Field K (2014) Hierarchy of root functional trait values and plasticity drive early-stage competition for water and phosphorus among grasses. Funct Ecol 28:1030–1040
Fransen B, de Kroon H (2001) Long-term disadvantages of selective root placement: root proliferation and shoot biomass of two perennial grass species in a 2-year experiment. J Ecol 89:711–722
Fransen B, de Kroon H, Berendse F (1998) Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia 115:351–358
Grime JP (1994) The role of plasticity in exploiting environmental heterogeneity. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants: ecophysiological processes above- and belowground. Academic Press, San Diego, pp 1–19
Grime JP, Crick JC, Rincon JE (1986) The ecological significance of plasticity. In: Jennings DH and Trewavas AJ (eds), Plasticity in Plants. Biologists Limited, pp. 5–29
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Jiao X, Lyu Y, Wu X, Li H, Cheng L, Zhang C, Yuan L, Jiang R, Jiang B, Rengel Z, Zhang F, Davies W, Shen J (2016) Grain production versus resource and environmental costs: towards increasing sustainability of nutrient use in China. J Exp Bot 67:4935–4949
Johnson CM, Ulrich A (1959) Analytical methods for use in plant analysis. University of California, Agricultural Experiment Station, Berkeley No 766:25–78
Kraft NJ, Godoy O, Levine JM (2015) Plant functional traits and the multidimensional nature of species coexistence. Proc Natl Acad Sci U S A 112:797–802
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103
Lambers H, Albornoz F, Kotula L, Laliberté E, Ranathunge K, Teste FP, Zemunik G (2017) How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil. https://doi.org/10.1007/s11104-017-3427-2
Li H, Ma Q, Li H, Zhang F, Rengel Z, Shen J (2014) Root morphological responses to localized nutrient supply differ among crop species with contrasting root traits. Plant Soil 376:151–163
Li HG, Liu J, Li G, Shen J, Bergström L, Zhang F (2015) Past, present, and future use of phosphorus in Chinese agriculture and its influence on phosphorus losses. Ambio 44:274–285
Li H, Wang X, Rengel Z, Ma Q, Zhang F, Shen J (2016) Root over-production in heterogeneous nutrient environment has no negative effects on Zea mays shoot growth in the field. Plant Soil 409:405–417
Li H, Liu B, McCormack M, Ma Z, Guo D (2017) Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol 216:1140–1150
Liu Y, Mi G, Chen F, Zhang J, Zhang F (2004) Rhizosphere effect and root growth of two maize (Zea mays L.) genotypes with contrasting P efficiency at low P availability. Plant Sci 167:217–223
Liu B, Li H, Zhu B, Koide RT, Eissenstat DM, Guo D (2015) Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol 208:125–136
Lyu Y, Tang H, Li H, Zhang F, Rengel Z, Whalley WR, Shen J (2016) Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Front Plant Sci 7:1939
MacArthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101:377–385
Mayfield MM, Levine JM (2010) Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol Lett 13:1085–1093
McNickle GG, Deyholos MK, Cahill JF (2016) Nutrient foraging behaviour of four co-occurring perennial grassland plant species alone does not predict behaviour with neighbours. Funct Ecol 30:420–430
Milla R, Morente-López J, Alonso-Rodrigo JM, Martín-Robles N, Chapin FS (2014) Shifts and disruptions in resource-use trait syndromes during the evolution of herbaceous crops. Proc R Soc Biol Sci 281:20141429
Mommer L, Visser EJ, van Ruijven J, de Caluwe H, Pierik R, de Kroon H (2011) Contrasting root behaviour in two grass species: a test of functionality in dynamic heterogeneous conditions. Plant Soil 344:347–360
Mommer L, Kirkegaard J, van Ruijven J (2016) Root–root interactions: towards a rhizosphere framework. Trends Plant Sci 21:209–217
Morris RA, Garrity DP (1993) Resource capture and utilization in intercropping: water. Field Crops Res 34:303–317
Nasto MK, Osborne BB, Lekberg Y, Asner GP, Balzotti CS, Porder S, Taylor PG, Townsend AR, Cleveland CC (2017) Nutrient acquisition, soil phosphorus partitioning and competition among trees in a lowland tropical rain forest. New Phytol 214:1506–1517
Neumann G (2006) Quantitative determination of acid phosphatase activity in the rhizosphere and on the root surface. In: Jones, D.L. (Eds.), 4.2 Biochemistry. In: Luster, J., Finlay, R. (Eds.), Handbook of Methods used in Rhizosphere Research - Online Edition
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MD, Lambers H (2006) Triticum aestivum shows a greater biomass response to a supply of aluminium phosphate than Lupinus albus, despite releasing fewer carboxylates into the rhizosphere. New Phytol 169:515–524
Prieto I, Violle C, Barre P, Durand JL, Ghesquiere M, Litrico I (2015) Complementary effects of species and genetic diversity on productivity and stability of sown grasslands. Nat Plant 1:15033
Richardson A, Hadobas P, Hayes J (2000) Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant Cell Environ 23:397–405
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156
Schmidt JE, Bowles TM, Gaudin AC (2016) Using ancient traits to convert soil health into crop yield: impact of selection on maize root and rhizosphere function. Front Plant Sci 7:373
Semchenko M, Lepik A, Abakumova M, Zobel K (2017) Different sets of belowground traits predict the ability of plant species to suppress and tolerate their competitors. Plant Soil. https://doi.org/10.1007/s11104-017-3282-1
Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156:997–1005
Shen J, Li C, Mi G, Li L, Yuan L, Jiang R, Zhang F (2013) Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China. J Exp Bot 64:1181–1192
Tilman D (1982) Resource competition and community structure. Princeton University Press
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and Genetical aspects of mycorrhizae. INRA Press, Paris, pp 217–221
Turner BL, Cade-Menun BJ, Condron LM, Newman S (2005) Extraction of soil organic phosphorus. Talanta 66:294–306
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Wang XX, Hoffland E, Feng G, Kuyper TW (2017) Phosphate uptake from phytate due to hyphae-mediated phytase activity by arbuscular mycorrhizal maize. Front Plant Sci 8:684
Wen Z, Li H, Shen J, Rengel Z (2017) Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant Soil 416:377–389
Wissuwa M, Mazzola M, Picard C (2009) Novel approaches in plant breeding for rhizosphere-related traits. Plant Soil 321:409–430
Wood SA, Karp DS, DeClerck F, Kremen C, Naeem S, Palm CA (2015) Functional traits in agriculture: agrobiodiversity and ecosystem services. Trends Ecol Evol 30:531–539
Zhang D, Zhang C, Tang X, Li H, Zhang F, Rengel Z, Whalley WR, Davies W, Shen J (2016) Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol 209:823–831
Zuppinger-Dingley D, Schmid B, Petermann JS, Yadav V, De Deyn GB, Flynn DF (2014) Selection for niche differentiation in plant communities increases biodiversity effects. Nature 515:108–111
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31772402, 31330070), National Key Research and Development Program of China (2016YFE0101100, 2017YFD0200200) and the Innovative Group Grant of the National Science Foundation of China (31421092). ZR is supported by Australian Research Council (DP160104434).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ismail Cakmak
Electronic supplementary material
ESM 1
(DOCX 67 kb)
Rights and permissions
About this article
Cite this article
Li, H., Zhang, D., Wang, X. et al. Competition between Zea mays genotypes with different root morphological and physiological traits is dependent on phosphorus forms and supply patterns. Plant Soil 434, 125–137 (2019). https://doi.org/10.1007/s11104-018-3616-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3616-7