Abstract
Background and aims
The current paradigm for phosphorus (P) fertilizers applied to calcareous soil is that almost entirely water soluble P fertilizers are efficient and sparingly soluble P fertilizers are not efficient P sources for crops. We hypothesize that this paradigm does not apply to recycled P fertilizers and that other P pools can explain the plant use of recycled P fertilizers on calcareous soil.
Methods
We applied 33P isotopic dilution method to evaluate recycled P fertilizers based on plant P uptake from fertilizer relative to plant uptake from a water soluble P reference fertilizer. The predictability of fertilizer effectiveness based on sequentially extracted P forms and X-ray diffraction pattern of recycled fertilizers derived from sewage sludge, human urine and organic waste was evaluated.
Results
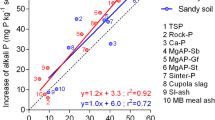
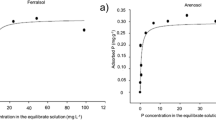
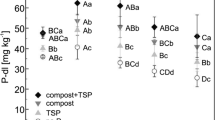
The plant experiments showed that tested recycled P fertilizers including compost were more effective than rock phosphate. The water insoluble P contained in urine based products was almost as effective as a fully water soluble P fertilizer. The tested recycled P fertilizers are characterized by complex P compounds differing in solubility which were so far not considered in the water and citric acid extraction methods. The fraction of resin- and NaHCO3 extractable fertilizer P explained effectiveness of P fertilizer applied to the calcareous and to an acidic soil.
Conclusion
We concluded that water solubility is not required when P forms in recycled products are comparable to reactions products of rock phosphate based fertilizers in soil. Alternatives to fully water soluble P fertilizers are available to supply P to crops grown on calcareous soil efficiently.





Similar content being viewed by others
Abbreviations
- AC:
-
Acidic soil
- AL:
-
Calcareous soil
- CSHP:
-
Calcium silicate hydrate phosphate
- DAMP:
-
Di-ammonium phosphate
- DCP:
-
Di-calcium phosphate
- MAP:
-
Mono-ammonium phosphate
- MCP:
-
Mono-calcium phosphate
- OWDd:
-
Organic waste based solid digestate, dried
- OWDdc:
-
Organic waste based solid digestate, composted
- Pdf:
-
Phosphorus derived from
- RP:
-
Rock phosphate
- SSA:
-
Sewage sludge ash
- STRU:
-
Struvite
- UCfN:
-
Urine calcium full nutrient
- UCP:
-
Urine calcium phosphate
- WSP:
-
Water soluble P
- XRD:
-
X-ray diffraction
References
Achat D, Daumer M-L, Sperandio M, Santellani A-C, Morel C (2014) Solubility and mobility of phosphorus recycled from dairy effluents and pig manures in incubated soils with different characteristics. Nutr Cycl Agroecosyst 99:1–15. https://doi.org/10.1007/s10705-014-9614-0
Adam C, Peplinski B, Michaelis M, Kley G, Simon FG (2009) Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag 29:1122–1128. https://doi.org/10.1016/j.wasman.2008.09.011
Agroscope F (2004) Referenzmethoden der Forschungsanstalten Agroscope, vol. 1–4. Forschungsanstalt Agroscope Reckenholz-Tänikon (ART) and Changins-Wädenswil (ACW), Zurich and Wädenswil
Andersson H, Bergström L, Ulén B, Djodjic F, Kirchmann H (2015) The role of subsoil as a source or sink for phosphorus leaching. J Environ Qual 44:535–544. https://doi.org/10.2134/jeq2014.04.0186
Bachmann S, Wentzel S, Eichler-Löbermann B (2011) Codigested dairy slurry as a phosphorus and nitrogen source for zea mays l. And amaranthus cruentus l. J Plant Nutr Soil Sci 174:908–915. https://doi.org/10.1002/jpln.201000383
Bailey JS, Beattie JAM, Kilpatrick DJ (1997) The diagnosis and recommendation integrated system (dris) for diagnosing the nutrient status of grassland swards: I. Model establishment. Plant Soil 197:127–135. https://doi.org/10.1023/a:1004236521744
Bekele T, Cino BJ, Ehlert PAI, Maas AA, Diest A (1983) An evaluation of plant-borne factors promoting the solubilization of alkaline rock phosphates. Plant Soil 75:361–378. https://doi.org/10.1007/bf02369971
Bell LC, Black CA (1970) Crystalline phosphates produced by interaction of orthophosphate fertilizers with slightly acid and alkaline soils1. Soil Sci Soc Am J 34:735–740. https://doi.org/10.2136/sssaj1970.03615995003400050020x
Berg U, Donnert D, Ehbrecht A, Bumiller W, Kusche I, Weidler PG, Nüesch R (2005) “Active filtration” for the elimination and recovery of phosphorus from waste water. Colloids Surf A Physicochem Eng Asp 265:141–148. https://doi.org/10.1016/j.colsurfa.2004.10.135
Bhuiyan MIH, Mavinic DS, Koch FA (2008) Thermal decomposition of struvite and its phase transition. Chemosphere 70:1347–1356. https://doi.org/10.1016/j.chemosphere.2007.09.056
Bolan NS, Hedley MJ (1989) Dissolution of phosphate rocks in soils. 1. Evaluation of extraction methods for the measurement of phosphate rock dissolution. Fertil Res 19:65–75. https://doi.org/10.1007/BF01054677
Bolan NS, Hedley MJ (1990) Dissolution of phosphate rocks in soils. 2. Effect of ph on the dissolution and plant availability of phosphate rock in soil with ph dependent charge. Fertil Res 24:125–134. https://doi.org/10.1007/BF01073580
Bolland M, Gilkes R (1988) The effectiveness of rock phosphate fertilisers in australian agriculture: a review. Aust J Exp Agric 28:655–668
Brod E, Øgaard A, Haraldsen T, Krogstad T (2015) Waste products as alternative phosphorus fertilisers part ii: predicting p fertilisation effects by chemical extraction. Nutr Cycl Agroecosyst 103:187–199. https://doi.org/10.1007/s10705-015-9731-4
Brod E, Øgaard AF, Krogstad T, Haraldsen TK, Frossard E, Oberson A (2016) Drivers of phosphorus uptake by barley following secondary resource application. Front Nutr 3:12. https://doi.org/10.3389/fnut.2016.00012
Brookes PC (1982) Correction for seed-phosphorus effects in l-value determinations. J Sci Food Agric 33:329–335. https://doi.org/10.1002/jsfa.2740330405
Bünemann EK, Bossio DA, Smithson PC, Frossard E, Oberson A (2004a) Microbial community composition and substrate use in a highly weathered soil as affected by crop rotation and p fertilization. Soil Biol Biochem 36:889–901. https://doi.org/10.1016/j.soilbio.2004.02.002
Bünemann EK, Smithson PC, Jama B, Frossard E, Oberson A (2004b) Maize productivity and nutrient dynamics in maize-fallow rotations in western kenya. Plant Soil 264:195–208. https://doi.org/10.1023/B:PLSO.0000047749.43017.fd
Capdevielle A, Sýkorová E, Biscans B, Béline F, Daumer M-L (2013) Optimization of struvite precipitation in synthetic biologically treated swine wastewater—determination of the optimal process parameters. J Hazard Mater 244–245:357–369. https://doi.org/10.1016/j.jhazmat.2012.11.054
Cornish PS (2009) Phosphorus management on extensive organic and low-input farms. Crop Pasture Sci 60:105–115. https://doi.org/10.1071/CP07134
Cross AF, Schlesinger WH (1995) A literature review and evaluation of the. Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64:197–214. https://doi.org/10.1016/0016-7061(94)00023-4
Degryse F, Baird R, da Silva RC, McLaughlin MJ (2017) Dissolution rate and agronomic effectiveness of struvite fertilizers – effect of soil ph, granulation and base excess. Plant Soil 410:139–152. https://doi.org/10.1007/s11104-016-2990-2
Duboc O, Santner J, Fard AG, Zehetner F, Tacconi J, Wenzel W (2017) Predicting phosphorus availability from chemically diverse conventional and recycling fertilizers. Sci Total Environ 599:1160–1170
Dyer B (1894) Xv.—on the analytical determination of probably available “mineral” plant food in soils. J Chem Soc Trans 65:115–167
Ehbrecht A, Schönauer S, Fuderer T, Schuhmann R (2011) P-recovery from sewage by seeded crystallisation in a pilot plant in batch mode technology. Water Sci Technol 63:339–344. https://doi.org/10.2166/wst.2011.061
EU (2003) Regulation (ec) no 2003/2003 of the european parliament and of the council of 13 october 2003 relating to fertilisers. Off J Eur Union 304:46
Fardeau J-C, Morel C, Boniface R (1988a) Pourquoi choisir la méthode olsen pour estimer le phosphore “assimilable” des sols? Agronomie 8:577–584
Fardeau J-C, Morel C, Jahiel M (1988b) Does long contact with the soil improve the efficiency of rock phosphate? Results of isotopic studies. Fertil Res 17:3–19. https://doi.org/10.1007/bf01050453
Faucon M-P, Houben D, Reynoird J-P, Mercadal-Dulaurent A-M, Armand R, Lambers H (2015) Chapter two - advances and perspectives to improve the phosphorus availability in cropping systems for agroecological phosphorus management. In: Sparks DL (ed) Advances in agronomy, vol 134. Academic Press, Cambridge, pp 51–79. https://doi.org/10.1016/bs.agron.2015.06.003
Frossard E, Morel JL, Fardeau JC, Brossard M (1994a) Soil isotopically exchangeable phosphorus: a comparison between e and l values. Soil Sci Soc Am J 58:846–851. https://doi.org/10.2136/sssaj1994.03615995005800030031x
Frossard E, Tekely P, Grimal JY (1994b) Characterization of phosphate species in urban sewage sludges by high-resolution solid-state 31p nmr. Eur J Soil Sci 45:403–408. https://doi.org/10.1111/j.1365-2389.1994.tb00525.x
Frossard E, Brossard M, Hedley MJ, Metherell A (1995) Reactions controlling the cycling of p in soils. In: Tiessen H (ed) Phosphorus in the global environment. John Wiley & Sons, Hoboken, pp 107–137
Frossard E, Sinaj S, Dufour P (1996a) Phosphorus in urban sewage sludges as assessed by isotopic exchange. Soil Sci Soc Am J 60:179–182. https://doi.org/10.2136/sssaj1996.03615995006000010029x
Frossard E, Sinaj S, Zhang L-M, Morel JL (1996b) The fate of sludge phosphorus in soil-plant systems. Soil Sci Soc Am J 60:1248–1253. https://doi.org/10.2136/sssaj1996.03615995006000040041x
Frossard E, Skrabal P, Sinaj S, Bangerter F, Traore O (2002) Forms and exchangeability of inorganic phosphate in composted solid organic wastes. Nutr Cycl Agroecosyst 62:103–113. https://doi.org/10.1023/A:1015596526088
Frost RL, Weier ML, Erickson K (2004) Thermal decomposition of struvite. J Therm Anal Calorim 76:1025–1033. https://doi.org/10.1023/B:JTAN.0000032287.08535.b3
Gallet A, Flisch R, Ryser J-P, Frossard E, Sinaj S (2003a) Effect of phosphate fertilization on crop yield and soil phosphorus status. J Plant Nutr Soil Sci 166:568–578. https://doi.org/10.1002/jpln.200321081
Gallet A, Flisch R, Ryser J-P, Nösberger J, Frossard E, Sinaj S (2003b) Uptake of residual phosphate and freshly applied diammonium phosphate by lolium perenne and trifolium repens. J Plant Nutr Soil Sci 166:557–567. https://doi.org/10.1002/jpln.200321075
Hedley M, McLaughlin M (2005) Reactions of phosphate fertilizers and by-products in soils. In: Sims JT, Sharpley AN (eds) Phosphorus: agriculture and the environment. Agronomy Monograph. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, pp 181–252. https://doi.org/10.2134/agronmonogr46.c7
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976. https://doi.org/10.2136/sssaj1982.03615995004600050017x
Hinsinger P et al (2011) Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant Soil 348:29–61. https://doi.org/10.1007/s11104-011-0903-y
House WA (1999) The physico-chemical conditions for the precipitation of phosphate with calcium. Environ Technol 20:727–733. https://doi.org/10.1080/09593332008616867
Kabbe C (2015) Technical factsheets. http://p-rex.eu
Keller L, Dollase WA (2000) X-ray determination of crystalline hydroxyapatite to amorphous calcium-phosphate ratio in plasma sprayed coatings. J Biomed Mater Res 49:244–249. https://doi.org/10.1002/(SICI)1097-4636(200002)49:2<244::AID-JBM13>3.0.CO;2-H
Kratz S, Schick J, Øgaard AF (2016) P solubility of inorganic and organic p sources. In: Schnug E, De Kok LJ (eds) Phosphorus in agriculture: 100% zero. Springer Netherlands, Dordrecht, pp 127–154. https://doi.org/10.1007/978-94-017-7612-7_7
Lemming C, Oberson A, Hund A, Jensen LS, Magid J (2016) Opportunity costs for maize associated with localised application of sewage sludge derived fertilisers, as indicated by early root and phosphorus uptake responses. Plant Soil 406(1–2):201–217
Lindsay WL, Frazier AW, Stephenson HF (1962) Identification of reaction products from phosphate fertilizers in soils. Soil Sci Soc Am J 26:446–452. https://doi.org/10.2136/sssaj1962.03615995002600050013x
Lombi E, McLaughlin MJ, Johnston C, Armstrong RD, Holloway RE (2004) Mobility and lability of phosphorus from granular and fluid monoammonium phosphate differs in a calcareous soil. Soil Sci Soc Am J 68:682–689. https://doi.org/10.2136/sssaj2004.6820
Lombi E, McLaughlin MJ, Johnston C, Armstrong RD, Holloway RE (2005) Mobility, solubility and lability of fluid and granular forms of p fertiliser in calcareous and non-calcareous soils under laboratory conditions. Plant Soil 269:25–34. https://doi.org/10.1007/s11104-004-0558-z
Mackay AD, Syers JK (1986) Effect of phosphate, calcium, and ph on the dissolution of a phosphate rock in soil. Fertil Res 10:175–184. https://doi.org/10.1007/BF01074371
Massey MS, Davis JG, Ippolito JA, Sheffield RE (2009) Effectiveness of recovered magnesium phosphates as fertilizers in neutral and slightly alkaline soils. Agron J 101:323–329. https://doi.org/10.2134/agronj2008.0144
Mattingly G (1975) Labile phosphate in soils. Soil Science 119:369–375
Moody P, Edwards D, Bell L (1995) Effect of banded fertilizers on soil solution composition and short-term root-growth .2. Mono-ammonium and di-ammonium phosphates. Soil Res 33:689–707. https://doi.org/10.1071/SR9950689
Morel C, Fardeau JC (1989) The uptake by crops of fresh and residual phosphatic fertilizers by simultaneous measurements with 32p and 33p. Int J Radiat Appl Instrum Part A 40:273–278. https://doi.org/10.1016/0883-2889(89)90217-7
Morel C, Fardeau JC (1991) Phosphorus bioavailability of fertilizers: a predictive laboratory method for its evaluation. Fertil Res 28:1–9. https://doi.org/10.1007/bf01048850
Nanzer S, Oberson A, Berger L, Berset E, Hermann L, Frossard E (2014a) The plant availability of phosphorus from thermo-chemically treated sewage sludge ashes as studied by 33p labeling techniques. Plant Soil 377(1–2):439–456
Nanzer S, Oberson A, Huthwelker T, Eggenberger U, Frossard E (2014b) The molecular environment of phosphorus in sewage sludge ash: implications for bioavailability. J Environ Qual 43:1050–1060. https://doi.org/10.2134/jeq2013.05.0202
Oberson A, Tagmann H, Langmeier M, Dubois D, Mäder P, Frossard E (2010) Fresh and residual phosphorus uptake by ryegrass from soils with different fertilization histories. Plant Soil 334:391–407. https://doi.org/10.1007/s11104-010-0390-6
Ohno T, Zibilske LM (1991) Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci Soc Am J 55:892–895. https://doi.org/10.2136/sssaj1991.03615995005500030046x
Okano K et al (2013) Novel technique for phosphorus recovery from aqueous solutions using amorphous calcium silicate hydrates (a-cshs). Water Res 47:2251–2259. https://doi.org/10.1016/j.watres.2013.01.052
Pypers P, Van Loon L, Diels J, Abaidoo R, Smolders E, Merckx R (2006) Plant-available p for maize and cowpea in p-deficient soils from the nigerian northern guinea savanna – comparison of l- and e-values. Plant Soil 283:251–264. https://doi.org/10.1007/s11104-006-0016-1
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Randall DG, Krähenbühl M, Köpping I, Larsen TA, Udert KM (2016) A novel approach for stabilizing fresh urine by calcium hydroxide addition. Water Res 95:361–369. https://doi.org/10.1016/j.watres.2016.03.007
Saggar S, Hedley MJ, White RE (1992) Development and evaluation of an improved soil test for phosphorus: 1. The influence of phosphorus fertilizer solubility and soil properties on the extractability of soil p. Fertil Res 33:81–91. https://doi.org/10.1007/BF01058012
Salimi MH, Heughebaert JC, Nancollas GH (1985) Crystal growth of calcium phosphates in the presence of magnesium ions. Langmuir 1:119–122. https://doi.org/10.1021/la00061a019
Saunders WMH, Williams EG (1955) Observations on the determination of total organic phosphorus in soils. J Soil Sci 6:254–267. https://doi.org/10.1111/j.1365-2389.1955.tb00849.x
Schnug E, Haneklaus SH (2016) The enigma of fertilizer phosphorus utilization. In: Schnug E, De Kok LJ (eds) Phosphorus in agriculture: 100% zero. Springer Netherlands, Dordrecht, pp 7–26. https://doi.org/10.1007/978-94-017-7612-7_2
Sinaj S, Traore O, Frossard E (2002) Effect of compost and soil properties on the availability of compost phosphate for white clover (trifolium repens l.) Nutr Cycl Agroecosyst 62:89–102. https://doi.org/10.1023/A:1015128610158
Southam DC, Lewis TW, McFarlane AJ, Borrmann T, Johnston JH (2008) Calcium–phosphorus interactions at a nano-structured silicate surface. J Colloid Interface Sci 319:489–497. https://doi.org/10.1016/j.jcis.2007.12.012
Spohn M, Ermak A, Kuzyakov Y (2013) Microbial gross organic phosphorus mineralization can be stimulated by root exudates – a 33p isotopic dilution study. Soil Biol Biochem 65:254–263. https://doi.org/10.1016/j.soilbio.2013.05.028
Stutter MI (2015) The composition, leaching, and sorption behavior of some alternative sources of phosphorus for soils. Ambio 44:207–216. https://doi.org/10.1007/s13280-014-0615-7
Suchanek WL, Byrappa K, Shuk P, Riman RE, Janas VF, TenHuisen KS (2004) Mechanochemical-hydrothermal synthesis of calcium phosphate powders with coupled magnesium and carbonate substitution. J Solid State Chem 177:793–799. https://doi.org/10.1016/j.jssc.2003.09.012
Syers JK, Mackay AD, Brown MW, Currie LD (1986) Chemical and physical characteristics of phosphate rock materials of varying reactivity. J Sci Food Agric 37:1057–1064. https://doi.org/10.1002/jsfa.2740371102
Syers JK, Johnston AE, Curtin D (2008) Efficiency of soil and fertilizer phosphorus use - reconciling changing concepts of soil phosphorus behaviour with agronomic information, vol 18. FAO, Rome
Tiessen H, Moir J (1993) Characterisation of available p by sequential extraction. CRC press Inc, Boca Raton
Traoré O, Sinaj S, Frossard E, Van De Kerkhove JM (1999) Effect of composting time on phosphate exchangeability. Nutr Cycl Agroecosyst 55:123–131. https://doi.org/10.1023/A:1009828927161
UNIDO, IFDC (1998) Fertilizer manual, 3rd edn. Kluwer Academic Publishers, Dordrecht and Muscle Shoals Accessed from http://nla.gov.au/nla.cat-vn1864349
Vogel C, Kohl A, Adam C (2011) Spectroscopic investigation in the mid- and far-infrared regions of phosphorus fertilizers derived from thermochemically treated sewage sludge ash. Appl Spectrosc 65:265–271
Wang L, Nancollas GH (2008) Calcium orthophosphates: crystallization and dissolution. Chem Rev 108:4628–4669. https://doi.org/10.1021/cr0782574
Williams C (1971) Reaction of surface-applied superphosphate with soil. Ii. Movement of the phosphorus and sulphur into the soil. Soil Res 9:95–106
WRB IWG (2014) World reference base for soil resources 2014 - international soil classification system for naming soils and creating legends for soil maps. FAO, Rome
van der Zee SEATM, Gjaltema A, van Riemsdijk WH, de Haan FAM (1992) Simulation of phosphate transport in soil columns. Ii. Simulation results. Geoderma 52:111–132. https://doi.org/10.1016/0016-7061(92)90078-L
Acknowledgements
We acknowledge the help of Brian Sinnet from Eawag with XRD analysis and Laurie Mauclaire-Schönholzer, Iris Huber and Eric Vogelsanger for their help with analyses and the plant growth experiments. We thank Jacques Fuchs from FiBL Switzerland for having provided the contact to the biogas plant in Pratteln, Switzerland. We also thank Wolfgang Ewert and Andreas Lengemann from Berliner Wasserbetriebe Wassmannsdorf, Germany, and Anke Ehbrecht from the Karlsruher Institute of Technology (KIT) for providing their recycled P products. Finally, we acknowledge the financial support of the CORE Organic II Funding Bodies, being partners of the FP7 ERA-Net project, CORE Organic II (Coordination of European Transnational Research in Organic Food and Farming systems, project no. 249667). We also thank N.J. Barrow and the three anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: N. Jim Barrow
Electronic supplementary material
ESM 1
(DOCX 297 kb)
Rights and permissions
About this article
Cite this article
Meyer, G., Frossard, E., Mäder, P. et al. Water soluble phosphate fertilizers for crops grown in calcareous soils – an outdated paradigm for recycled phosphorus fertilizers?. Plant Soil 424, 367–388 (2018). https://doi.org/10.1007/s11104-017-3545-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3545-x