Abstract
Background and aims
Many rhizobacteria promote plant growth by producing hormones that stimulate the development of plant root system and increase plant biomass. The aim of this study was to investigate the growth promotion activity of the bacterial strain Martelella endophytica YC6887 and elucidate the signaling pathways potentially involved in Arabidopsis interaction with M. endophytica YC6887.
Methods
The growth regulation was evaluated by inoculation of strain YC6887 with wild-type Arabidopsis Col-0 seedlings and mutants defective in auxin aux1-7, axr4-2, eir1-1, ethylene ein2-1, etr1-3, jasmonic acid signaling jar1, and root hair deficient mutant rhd6. The auxin response was further determined by using transgenic line DR5::GUS and a polar auxin transport inhibitor, 1-N-naphthylphthalamic acid (NPA).
Results
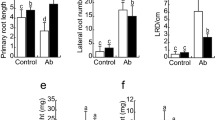
M. endophytica YC6887 increased the number of lateral roots and plant biomass of Arabidopsis by producing phenylacetic acid. The growth promotion and improved lateral root development by the bacterium decreased in the auxin related mutants, whereas the ethylene and jasmonic acid mutants had a wild type response. The strain YC6887 increased root hair density in wild type Col-0 and recovered the root hair forming ability in root hair deficient mutant rhd6. Moreover, strain YC6887 treatment showed distinct response in DR5::GUS transgenic line compared to the control. Strain YC6887 lost its growth-promoting activity in the presence of NPA, an auxin transport inhibitor. This indicated that strain YC6887 activated the auxin signaling mechanism.
Conclusions
Our results showed that M. endophytica YC6887 promoted plant growth in terms of plant biomass and root system development. Arabidopsis root system development upon M. endophytica YC6887 colonization was dependent on auxin signaling, but independent of ethylene and jasmonic acid signaling.







Similar content being viewed by others
Abbreviations
- IAA:
-
(Indole-3-acetic acid)
- LRP:
-
(Lateral root primordia)
- NPA:
-
(1-N-Naphthylphthalamic acid)
- PAA:
-
(Phenylacetic acid)
References
Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815
Arkhipova T, Veselov S, Melentiev A, Martynenko E, Kudoyarova G (2005) Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272:201–209
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Bibi F, Yasir M, Song GC, Lee SY, Chung YR (2012) Diversity and characterization of endophytic bacteria associated with tidal flat plants and their antagonistic effects on omycetes plant pathogens. Plant Pathol J 28:20–31
Bibi F, Chung EJ, Khan A, Jeon CO, Chung YR (2013) Martelella endophytica sp. nov., an antifungal bacterium associated with a halophyte. Int J Syst Evol Microbiol 63:2914–2919
Cassan F, Maiale S, Masciarelli O, Vidal A, Luna V, Ruiz O (2009) Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol 45:12–19
Chowdhury SP, Uhl J, Grosch R, Alquéres S, Pittroff S, Dietel K, Schmitt-Kopplin P, Borriss R, Hartmann A (2015) Cyclic lipopeptides of Bacillus amyloliquefaciens subsp. plantarum colonizing the Lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Mol Plant-Microbe Interact 28:984–995
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005a) Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Compant S, Reiter B, Sessitsch A, Nowak J, Clement C, Barka EA (2005b) Endophytic colonization of Vitis vinifera L. by plant growth promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microbiol 71:1685–1693
Contesto C, Milesi S, Mantelin S, Zancarini A, Desbrosses G, Varoquaux F, Bellini C, Kowalczyk M, Touraine B (2010) The auxin-signaling pathway is required for the lateral root response of Arabidopsis to the rhizobacterium Phyllobacterium brassicacearum. Planta 232:1455–1470
Contreras-Cornejo HA, Macias-Rodriguez L, Cortes-Penagos C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149:1579–1592
Contreras-Cornejo HA, Macias-Rodriguez L, Alfaro-Cuevas R, López-Bucio J (2014) Trichoderma spp. improves the growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production and Na+ elimination through root exudates. Mol Plant-Microbe Interact 276:503–514
Costacurta A, Vanderleyden J (1995) Synthesis of phytohormones by plant associated bacteria. Crit Rev Microbiol 21:1–18
Dashti N, Zhang F, Hynes R, Smith DL (1998) Plant growth promoting rhizobacteria accelerate nodulation and increase nitrogen fixation activity by field grown soybean [Glycine max (L.) Merr.] under short season conditions. Plant Soil 200:205–213
Davies, PJ (2004) Plant hormones: biosynthesis, signal transduction, action. The plant hormone: their nature, occurrence and function. (Ed.) Dordrecht, the Netherlands: Kluwer Academic Publishers.
Dharmasiri S, Swarup R, Mockaitis K, Dharmasiri N, Singh SK, Kowalchyk M, Marchant A, Milli S, Sandberg G, Bennet MJ, Estelle M (2006) AXR4 is required for localization of the auxin influx facilitator Aux1. Science 312:1218–1220
Dobbelaere S, Croonenborghs A, Thys A, Broek AV, Vanderleyden J (1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:153–162
Felten J, Kohler A, Morin E, Bhalerao RP, Palme K, Martin F, Ditengou FA, Legué V (2009) The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol 151:1991–2005
Galland M, Gamet L, Varoquaux F, Touraine B, Touraine B, Desbrosses G (2012) The ethylene pathway contributes to root hair elongation induced by the beneficial bacteria Phyllobacterium brassicacearum STM196. Plant Sci 190:74–81
Gray WM (2004) Hormonal regulation of plant growth and development. PLoS Biol 2:e311
Gruber BD, Giehl RF, Friedel S, Wiren VN (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163:161–179
Grunewald W, Noorden GV, Isterdael GV, Beeckman T, Gheysen G, Mathesius U (2009) Manipulation of auxin transport in plant roots during Rhizobium symbiosis and nematode parasitism. Plant Cell 21:2553–2562
Hobbie LJ (2006) Auxin and cell polarity: the emergence of AXR4. Trends Plant Sci 11:517–518
Hobbie L, Estelle M (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define root initiation. Plant J 7:211–220
Hwang BK, Lim SW, Kim BS, Lee JY, Moon SS (2001) Isolation and in vivo and invitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidis. Appl Environ Microbiol 67:3739–3745
Ivanchenko MG, Muday GK, Dubrovsky JG (2008) Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55:335–347
Kertesz MA, Mirleau P (2004) The role of soil microbes in plant sulphur nutrition. J Exp Bot 55:1939–1945
Lacoul P, Freedman B (2006) Environmental influences on aquatic plants in freshwater ecosystems. Environ Rev 14:89–136
Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18:450–458
Lee RDW, Cho HT (2013) Auxin, the organizer of the hormonal/environmental signals for root hair growth. Front Plant Sci 4:1–7
Ljung K (2013) Auxin metabolism and homeostasis during plant development. Development 140:943–950
López-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287
López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodríguez R, Macías-Rodríguez LI, Valencia-Cantero E (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin-and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant Microbe Interact 20:207–217
Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root specific protein involved in auxin transport is required for gravitotropism in Arabidopsis thaliana. Genes Dev 12:2175–2187
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44
Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14:589–597
Masucci JD, Shieffelbein JW (1994) The rhd6 mutation of Arabidopsis thaliana alters root hair initiation through an auxin and ethylene associated process. Plant Physiol 106:1335–1346
Mathesius U (2008) Goldacre paper: auxin: at the root of nodule development? Funct Plant Biol 35:651–668
Meldau DG, Meldau S, Hoang LH, Underberg S, Wünsche H, Baldwin IT (2013) Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp. B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 25:2731–2747
Ortiz-Castro R, Valancia-Cantero E, López-Bucio J (2008) Plant growth promotion by Bacillus megaterium involve cytokinin signaling. Plant Signal Behav 3:263–265
Ortiz-Castro R, Díaz-Pérez C, Martínez-Trujillo M, Rosa E, Campos-García J, López-Bucio J (2011) Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc Natl Acad Sci 108:7253–7258
O’sullivan DJ, O’Gara F (1992) Traits of fluorescent pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev 56:662–676
Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14:399–408
Pickett FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94:1462–1466
Pitts RJ, Cernac A, Estelle M (1998) Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J 16:553–560
Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S (2002) Auxin and ethylene response interactions during Arabidopsis root hair development dissected by Auxin influx modulators. Plant Physiol 130:1908–1917
Ramos Solano B, Barriuso Maicas J, Pereyra De La Iglesia MT, Domenech J, Gutiérrez Mañero FJ (2008) Systemic disease protection elicited by plant growth promoting rhizobacteria strains: relationship between metabolic responses, systemic disease protection, and biotic elicitors. Phytopathology 98:451–457
Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14:435–443
Ribaudo CM, Krumpholz EM, Cassán FD, Bottini R, Cantore ML, Curá JA (2006) Azospirillum sp. promotes root hair development in tomato plants through a mechanism that involves ethylene. J Plant Growth Regul 25:175–185
Richardson AE, Barea JM, Mcneill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant promotion by microorganisms. Plant Soil 321:305–339
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci 100:4927–4932
Sgroy V, Cassán F, Masciarelli O, Del Papa MF, Lagares A, Luna V (2009) Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl Microbiol Biotechnol 85:371–381
Shin DS, Myung SP, Jung S, Myoung SL, Lee KH, Kyung SB, Seung BK (2007) Plant growth-promoting potential of endophytic bacteria isolated from roots of coastal sand dune plants. J Microbiol Biotechnol 17:1361–1368
Somers E, Ptacek D, Gysegom P, Srinivasan M, Vanderleyden J (2005) Azospirillum brasilence produces the auxin like phenylacetic acid by usin g the key enzyme for indole-3-acetic acid biosynthesis. Appl Environ Microbiol 71:1803–1810
Splivallo R, Fischer U, Göbel C, Feussner I, Karlovsky P (2009) Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol 150:2018–2029
Sugawara S, Mashiguchi K, Tanaka K, Hishiyama S, Sakai T, Hanada K, Tsujimura KK, Yu H, Dai X, Takebayashi Y, Kamiya NK, Kakimoto T, Kawaide H, Natsume M, Estelle M, Zhao Y, Hayashi KI, Kamiya Y Kasahara, H (2015) Distinct characteristics of indole-3-acetic acid and phenylacetic acid, two common auxins in plants. Plant Cell Physiol 56:1641–1654
Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, Zhou W, Chen R, Li X, Tietz O, Wu X, Cohen JD, Palme K, Li C (2009) Arabidopsis ASA1 is important for jasmonate mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21:1495–1511
Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39:792–796
Tanimoto M, Roberts K, Dolan L (1995) Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J 8:943–948
Tian H, De Smet I, Ding Z (2014) Shaping a root system: regulating lateral versus primary root growth. Trends Plant Sci 19:426–431
Timmusk S, Nicander B, Granhall U, Tillberg E (1999) Cytokinin production by Paenibacillus polymyxa. Soil Biol Biochem 31:1847–1852
Timmusk S, Grantcharova N, Wagner EGH (2005) Paenibacillus polymyxa invades plant roots and forms biofilms. Appl Environ Microbiol 71:7292–7300
Walker V, Bertrand C, Bellvert F, Moenne-Loccoz Y, Bally R, Comte G (2011) Host plant secondary metabolite profiling shows a complex, strain dependent response of maize to plant growth promoting rhizobacteria of the genus Azospirillum. New Phytol 189:494–506
Weyen N, Van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009) Exploiting plant-microbe partnership to improve biomass production and remediation. Trends Biotechnol 27:591–598
Wightman F, Lighty DL (1982) Identification of phenylacetic acid as a natural auxin in the shoots of higher plants. Physiol Plant 55:17–24
Xie SS, Wu HJ, Zhang HY, Wu LM, Zhu QQ, Gao XW (2014) Plant growth promotion by spermidine-producing Bacillus subtilis OKB105. Mol Plant-Microbe Interact 27:655–663
Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse CM (2013) Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol 162:304–318
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, Faraq MA, Ryu CM, Allen R, Melo IS, Paré PW (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851
Zhu C, Gan L, Shen Z, Xia K (2006) Interaction between jasmonates and ethylene in the regulation of root hair development in Arabidopsis. J Exp Bot 57:1299–1308
Acknowledgments
This work was supported by the Brain Korea (BK) 21 Plus project, the Ministry of Education, Science and Technology, Republic of Korea and was partially funded by a Research and Business Development grant provided by the Ministry of Food, Agriculture, Forestry and Fisheries, Korea (no. 808015–3). We thank Jae Yean Kim from Gyeongsang National University for providing the Arabidopsis mutants, ein2-1 and DR5::GUS and also thank Malcolm J. Bennett, University of Nottingham, for providing the auxin mutants, aux1-7, axr4-2 and eir1-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jesus Mercado-Blanco.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
The roots of Arabidopsis (Col-0) plants were drenched with the suspension of Martelella endophytica YC6887 in buffer solution (10 mM MgSO4) at 2 × 106, 5 × 107, and 5 × 108 CFU mL−1. Buffer was used as a control. Representative plants showed growth one week after inoculation of the strain YC6887. (DOC 4921 kb)
Fig. S2
Effect of PAA on root system development of auxin mutants aux1-7, axr4-2, and eir1-1. a lateral root number/seedling and b primary root length. Black bars represent untreated seedlings control. Gray bars indicate phenylacetic acid treated seedlings. Means ± standard error within bars are significantly different (Duncan’s test; P < 0.05). (DOC 59 kb)
Fig. S3
Effect of PAA and IAA on primary root length of Arabidopsis. a nano molar concentration and b micro molar concentration. Black circle shows different concentration of PAA. White circles shows IAA. (DOC 60 kb)
Fig. S4
Structure determination of phenylacetic acid, a chemical structure of phenylacetic acid, b 1H-NMR spectrum, c 13C-NMR spectrum and d EI-MS spectrum. (DOC 2305 kb)
Fig. S5
Effect of NPA (10 μM) on DR5::GUS transgenic lines. a control, b Martelella endophytica YC6887, c only NPA, and d NPA with M. endophytica YC6887 treated DR5::GUS transgenic line. LRP of DR5::GUS transgenic lines after Gus staining, e LRP of control seedlings, f LRP of PAA, and g LRP of M. endophytica YC6887 treated plants (scale bar = 100 μm). (DOC 3074 kb)
Fig. S6
Effect of PAA at different concentrations (4 μm and 8 μm) on primary root and LRP formation in DR5::GUS seedlings. a Primary root tip of control, b and c PPA treated DR5::GUS seedlings (scale bar = 100 μm), d LRP formation in control and PAA treated DR5::GUS seedlings (scale bar = 200 μm). (DOC 1145 kb)
Rights and permissions
About this article
Cite this article
Khan, A., Hossain, M.T., Park, H.C. et al. Development of root system architecture of Arabidopsis thaliana in response to colonization by Martelella endophytica YC6887 depends on auxin signaling. Plant Soil 405, 81–96 (2016). https://doi.org/10.1007/s11104-015-2775-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2775-z