Abstract
Background and aims
Chickpea rhizobia did not occur naturally in Australian cropping soils, necessitating inoculation at sowing. Now, after more than 30 years of chickpea cultivation using a single inoculant strain, CC1192, it is likely that chickpea rhizobia are established in 1.0–1.5 Mha cropping land. The aims of this study were to examine effects of the naturalised chickpea rhizobia on nodulation and productivity (total crop N, crop N fixed and grain yield) of commercial chickpea.
Methods
Soil was sampled from 26 fields to estimate chickpea rhizobial numbers, relate numbers to edaphic factors and years since previous chickpea crop, determine the proportions of CC1192 and novel strains using RAPD-PCR and subject a subset of novel strains from one site to 16S rRNA analysis. Nodules were harvested from 15 inoculated, commercial chickpea crops to determine occupancy by CC1192. The symbiotic effectiveness of a second subset of novel strains was assessed.
Results
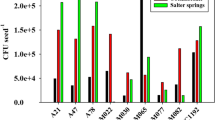
The mean number of rhizobia in the soils varied from log 0.08 to log 5.16 rhizobia g soil−1 with population size positively correlated with soil moisture content and negatively correlated with salt concentration (ECe). RAPD-PCR analysis of 570 strains of chickpea rhizobia isolated from the soils indicated only 14 % with molecular fingerprints similar to CC1192. Occupancy by CC1192 of nodules harvested from commercial crops ranged 0–100 %, with an average of 53 %. Occupancy by CC1192 declined by an average 17 % with each log unit increase in numbers of novel chickpea rhizobia.
Conclusions
We found no evidence that the novel mesorhizobia in the chickpea soils compromised N2 fixation or productivity of commercial chickpea crops, even though individual strains had generally reduced symbiotic effectiveness relative to CC1192.






Similar content being viewed by others
References
Ballard RA, Charman N, McInnes A, Davidson JA (2004) Size, symbiotic effectiveness and genetic diversity of field pea rhizobia (Rhizobium leguminosarum bv. viciae) populations in South Australian soils. Soil Biol Biochem 36:1347–1355
Barcellos FG, Menna P, Batista JSD, Hungria M (2007) Evidence of horizontal transfer of symbiotic genes from a Bradyrhizobium japonicum inoculant strain to indigenous diazotrophs Sinorhizobium (Ensifer) fredii and Bradyrhizobium elkanii in a Brazilian Savannah soil. Appl Environ Microb 732635–2643
Batista JSS, Hungria M, Barcellos FG, Ferreira MC, Mendes IC (2007) Variability in Bradyrhizobium japonicum and B. elkanii seven years after introduction of both the exotic microsymbiont and the soybean host in a cerrados soil. Microb Ecol 53:270–284
Beiko RG, Harlow TJ, Ragan MA (2005) Highways of gene sharing in prokaryotes. P Natl Acad Sci USA 102:14332–14337
Bottomley PJ, Jenkins MB (1983) Some characteristics of Rhizobium meliloti isolates from alfalfa fields in Oregon. Soil Sci Soc Am J 47:1153–1157
Bowman AM, Hebb DM, Munnich DJ, Brockwell J (1998) Rhizobium as a factor in the re-establishment of legume-based pastures on clay soils of the wheat belt of north-western New South Wales. Aust J Exp Agr 38:555–566
Brockwell J (1963) Accuracy of a plant-infection technique for counting populations of Rhizobium trifolii. Appl Microbiol 11:377–383
Brockwell J, Holliday RA, Pilka A (1988) Evaluation of the symbiotic nitrogen-fixing potential of soils by direct microbiological means. Plant Soil 108:163–170
Bullard GK, Roughley RJ, Pulsford DJ (2005) The legume inoculant industry and inoculant quality control in Australia: 1953–2003. Aust J Exp Agr 45:127–140
Bushby HVA, Marshall KC (1977a) Some factors affecting the survival of root-nodule bacteria on desiccation. Soil Biol Biochem 9:143–147
Bushby HVA, Marshall KC (1977b) Water status of rhizobia in relation to their susceptibility to desiccation and to their protection by montmorillonite. J Gen Microbiol 99:19–27
Chen LS, Figueredo A, Villani H, Michajluk J, Hungria M (2002) Diversity and symbiotic effectiveness of rhizobia isolated from field-grown soybean nodules in Paraguay. Biol Fert Soils 35:448–457
Corbin EJ, Brockwell J, Gault RR (1977) Nodulation studies on chickpea (Cicer arietinum). Aust J Exp Agr Anim Hus 17:126–134
Daniels I, Manning B, Pearce L (2002) Profile descriptions: district guidelines for managing soils in north-west NSW. NSW Agriculture, Sydney
Denton MD, Coventry DR, Bellotti WD, Howieson JG (2000) Distribution, abundance and symbiotic effectiveness of Rhizobium leguminosarum bv. trifolii from alkaline pasture soils in South Australia. Aust J Exp Agr 40:25–35
Drew EA, Ballard RA (2010) Improving N2 fixation from the plant down: compatability of Trifolium subterraneum L. cultivars with soil rhizobia can influence symbiotic performance. Plant Soil 327:261–277
Elias NV, Herridge DF (2014) Crop-available water and agronomic management, rather than nitrogen supply, primarily determine grain yield of commercial chickpea in northern New South Wales. Crop Pasture Sci 65:442–452 http://dx.doi.org/10.1017/CP13397
Evans J (2005) An evaluation of potential Rhizobium inoculant strains used for pulse production in acidic soils of south-east Australia. Aust J Exp Agr 45:257–268
Evans J, Hochman Z, Oconnor GE, Osborne GJ (1988) Soil acidity and Rhizobium - their effects on nodulation of subterranean clover on the slopes of southern New-South-Wales. Aust J Agr Res 39:605–618
FAOSTAT (2013) FAO Statistics Division, viewed on 15 November 2013, http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID = 567#ancor
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Ferreira MC, Hungria M (2002) Recovery of soybean inoculant strains from uncropped soils in Brazil. Field Crop Res 79:139–152
Ferreira MC, Andrade DS, Chueire LMO, Takemura SM, Hungria M (2000) Tillage method and crop rotation effects on the population sizes and diversity of bradyrhizobia nodulating soybean. Soil Biol Biochem 32:627–637
Furseth BJ, Conley SP, Ane JM (2012) Soybean response to soil rhizobia and seed-applied rhizobia inoculants in Wisconsin. Crop Sci 52:339–344
Gerding M, O’Hara GW, Howieson JG, Brau L (2014) Overcoming non-selective nodulation of Lessertia by soil-borne rhizobium in the presence of inoculant mesorhizobium. Plant Soil 380:117–132
Grange L, Hungria M, Graham PH, Martinez-Romero E (2007) New insights into the origins and evolution of rhizobia that nodulate common bean (Phaseolus vulgaris) in Brazil. Soil Biol Biochem 39:867–876
Hardarson G, Golbs M, Danso SK (1989) Nitrogen fixation in soybean (Glycine max L. Merrill) as affected by nodulation patterns. Soil Biol Biochem 21:783–787
Herridge D (1977) Carbon and nitrogen nutrition of two annual legumes. University of Western Australia, PhD
Herridge D (2008) Inoculation technology for legumes. In: Dilworth MJ, James EK, Sprent JI, Newton WE (eds) Nitrogen-fixing leguminous-symbioses. Springer, Dordrecht, The Netherlands, pp 77–109
Howieson J, Ballard R (2004) Optimising the legume symbiosis in stressful and competitive environments within southern Australia – some contemporary thoughts. Soil Biol Biochem 36:1261–1273
Hungria M, Campo RJ, Chueire LM, Grange L, Megias M (2001a) Symbiotic effectiveness of fast-growing rhizobial strains isolated from soybean nodules in Brazil. Biol Fert Soils 33:387–394
Hungria M, Chueire LM, Coca RG, Megias M (2001b) Preliminary characterization of fast growing rhizobial strains isolated from soybean nodules in Brazil. Soil Biol Biochem 33:1349–1361
Issa S, Wood M (1995) Multiplication and survival of chickpea and bean rhizobia in dry soils: the influence of strains, matric potential and soil texture. Soil Biol Biochem 27:785–792
Keatinge JDH, Beck DP, Materon LA, Yurtsever N, Karuc K, Altuntas S (1995) The role of rhizobial biodiversity in legume crop productivity in the west Asian highlands. IV. Rhizobium ciceri. Exp Agr 31:501–507
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kucey RMN, Hynes MF (1989) Populations of Rhizobium leguminosarum biovars phaseoli and viceae in fields after bean or pea in rotation with non legumes. Can J Microbiol 35:661–667
Kumar S, Tamura K, Nei M (2004) MEGA3: Integrated software for molecular evolutionary genetic analysis and sequence alignment. Brief Bioinform 5:150–163
Kyei-Boahen S, Slinkard AE, Walley FL (2002) Evaluation of rhizobial inoculation methods for chickpea. Agron J 94:851–859
Laguerre G, Louvrier P, Allard MR, Amarger N (2003) Compatibility of rhizobial genotypes within natural populations of Rhizobium leguminosarum biovar viciae for nodulation of host legumes. Appl Environ Microb 69:2276–2283
Laranjo M, Alexandre A, Rivas R, Velazquez E, Young JPW, Oliveira S (2008) Chickpea rhizobia symbiosis genes are highly conserved across multiple Mesorhizobium species. FEMS Microbiol Ecol 66:391–400
L’Taief B, Sifi B, Gtari M, Zaman-Allah M, Lachaal M (2007) Phenotypic and molecular characterization of chickpea rhizobia isolated from different areas of Tunisia. Can J Microbiol 53:427–434
Marshall TJ (1947) Mechanical composition of soil in relation to field descriptions of texture. Bulletin No. 224. Council for Scientific and Industrial Research Bulletin, Melbourne, Australia
Mothapo NV, Grossman JM, Sooksa-nguan T, Maul J, Brauer SL, Shi W (2013) Cropping history affects nodulation and symbiotic efficiency of distinct hairy vetch (Vicia villosa Roth.) genotypes with resident soil rhizobia. Biol Fert Soils 49:871–879
Nandasena KG, O'Hara GW, Tiwari RP, Howieson JG (2006) Rapid in situ evolution of nodulating strains for Biserrula pelecinus L. through lateral transfer of a symbiosis island from the original mesorhizobial inoculant. Appl Environ Microbiol 72:7365–7367
Nandasena KG, O'Hara GW, Tiwari RP, Sezmis E, Howieson JG (2007) In situ lateral transfer of symbiosis islands results in rapid evolution of diverse competitive strains of mesorhizobia suboptimal in symbiotic nitrogen fixation on the pasture legume Biserrula pelecinus L. Environ Microbiol 9:2496–2511
Nour SM, Cleyetmarel JC, Beck D, Effosse A, Fernandez MP (1994) Genotypic and phenotypic diversity of Rhizobium isolated from chickpea (Cicer arietinum L). Can J Microbiol 40:345–354
Rayment GE, Higginson FR (1992) The Australian laboratory handbook of soil and water chemical methods. Inkata Press, Sydney
Revellin C, Pinochet X, Beauclair P, Catroux G (1996) Influence of soil properties and soya bean cropping history on the Bradyrhizobium japonicum population in some French soils. Eur J Soil Sci 47:505–510
Richardson AE, Viccars LA, Watson JM, Gibson AH (1995) Differentiation of Rhizobium strains using the polymerase chain reaction with random and directed primers. Soil Biol Biochem 27:515–524
Rivas R, Laranjo M, Mateos PF, Oliveira S, Martinez-Molina E, Velazquez E (2007) Strains of Mesorhizobium amorphae and Mesorhizobium tianshanense, carrying symbiotic genes of common chickpea endosymbiotic species, constitute a novel biovar (ciceri) capable of nodulating Cicer arietinum. Lett Appl Microbiol 44:412–418
Rodrigues CS, Laranjo M, Oliveira S (2006) Effect of heat and pH stress in the growth of chickpea mesorhizobia. Curr Microbiol 53:1–7
Rupela OP, Saxena MC (1987) Nodulation and nitrogen fixation in chickpea. The chickpea. CAB International, Wallingford, UK, In, pp 191–206
Rupela OP, Toomsan B, Mittal S, Dart PJ, Thompson JA (1987) Chickpea rhizobium populations: survey of influence of season, soil depth and cropping pattern. Soil Biol Biochem 19:247–252
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual 2nd Ed. Cold Spring Harbour Laboratory Press. Cold Spring Harbour, New York
Santos MA, Vargas MAT, Hungria M (1999) Characterization of soybean Bradyrhizobium strains adapted to the Brazilian savannas. FEMS Microbiol Ecol 30:261–272
Slattery JF, Coventry DR, Slattery WJ (2001) Rhizobial ecology as affected by the soil environment. Aust J Exp Agr 41:289–298
Slattery JF, Pearce DJ, Slattery WJ (2004) Effects of resident rhizobial communities and soil type on the effective nodulation of pulse legumes. Soil Biol Biochem 36:1339–1346
Slavich PG, Petterson GH (1993) Estimating the electrical conductivity of saturated paste extracts from 1:5 soil:water suspensions and texture. Aust J Soil Res 31:73–81
Soil Survey Staff (2010) Keys to soil taxonomy (11th Edition). USDANRCS, US Gov. Print. Office, Washington, DC, USA
Somasegaran P, Hoben HJ (1994) Handbook for rhizobia: methods in legume-Rhizobium technology. Springer, New York
Sperazza M, Moore JN, Hendrix MS (2004) High-resolution particle size analysis of naturally occurring very fine-grained sediment through laser diffractometry. J Sediment Res 74:736–743
Sullivan JT, Patrick HN, Lowther WL, Scott DB, Ronson CW (1995) Nodulating strains of Rhizobium loti arise through chromosomal symbiotic gene transfer in the environment. P Natl Acad Sci USA 92:8985–8989
Sullivan JT, Ronson CW (1998) Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. P Natl Acad Sci USA 95:5145–5149
Thies JE, Bohlool BB, Singleton PW Environmental effects on competition for nodule occupancy between introduced and indigenous rhizobia and among introduced strains. Can J Microbiol 38:493–500 (1992)
Thies JE, Holmes EM, Vachot A (2001) Application of molecular techniques to studies in Rhizobium ecology: a review. Aust J Exp Agr 41:299–319
Thies JE, Singleton PW, Benbohlool B (1991) Influence of the size of indigenous rhizobial populations on establishment and symbiotic performance of introduced rhizobia on field-grown legumes. Appl Environ Microbiol 57:19–28
Vachot-Griffin AM, Thies JE (2005) Fingerprinting the Australian inoculant mother cultures using refined PCR protocols yields beneficial inoculant management options. Aust J Exp Agr 45:141–150
Wadisirisuk P, Danso SKA, Hardarson G, Bowen GD (1989) Influence of Bradyrhizobium japonicum location and movement on nodulation and nitrogen fixation in soybeans. Appl Environ Microb 55:1711–1716
Weaver RW, Frederick LR (1974) Effect of inoculum rate on competitive nodulation of Gycine max L. Merrill. I. Greenhouse studies. Agron J 66:229–232
Woomer P, Bennett J, Yost R (1990) Overcoming the inflexibility of most-probable-number procedures. Agron J 82:349–353
Acknowledgments
We thank Dr Alison McInnes, formerly of the University of Western Sydney, for scientific and technical direction and Judith Gray, University of Western Sydney, for assistance with the RAPD-PCR analysis. We gratefully acknowledge Dr Kemanthie Nandasena, Centre for Rhizobium Studies (CRS), Murdoch University, Western Australia, for the 16S rRNA gene sequencing and for her considerable and unselfish assistance in interpreting the results We also thank Professor Graham O’Hara also from the CRS, Murdoch University, for providing additional interpretation of and comment on the sequencing data. We acknowledge the Australian Grains Research & Development Corporation (GRDC) for financial support for the post-graduate scholarship (NE) and project support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Euan K. James.
Rights and permissions
About this article
Cite this article
Elias, N.V., Herridge, D.F. Naturalised populations of mesorhizobia in chickpea (Cicer arietinum L.) cropping soils: effects on nodule occupancy and productivity of commercial chickpea. Plant Soil 387, 233–249 (2015). https://doi.org/10.1007/s11104-014-2298-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2298-z