Abstract
Aims
This study aimed to analyse the effect of phosphorus (P) nutritional status on wheat leaf surface properties, in relation to foliar P absorption and translocation.
Methods
Plants of Triticum aestivum cv. Axe were grown with three rates of root P supply (equivalent to 24, 8 and 0 kg P ha−1) under controlled conditions. Foliar P treatments were applied and the rate of drop retention, P absorption and translocation was measured. Adaxial and abaxial leaf surfaces were analysed by scanning and transmission electron microscopy. The contact angles, surface free energy and work-of-adhesion for water were determined.
Results
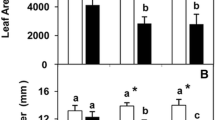
Wheat leaves are markedly non-wettable, the abaxial leaf side having some degree of water drop adhesion versus the strong repulsion of water drops by the adaxial side. The total leaf area, stomatal and trichome densities, cuticle thickness and contact angles decreased with P deficiency, while the work-of-adhesion for water increased. Phosphorous deficient plants failed to absorb the foliar-applied P.
Conclusions
Phosphorous deficiency altered the surface structure and functioning of wheat leaves, which became more wettable and had a higher degree of water drop adhesion, but turned less permeable to foliar-applied P. The results obtained are discussed within an agronomic and eco-physiological context.


Similar content being viewed by others
References
Ahmad I, Wainwright S (1976) Ecotype differences in leaf surface properties of Agrostis stolonifera from salt marsh, spray zone and inland habitats. New Phytol 76(2):361–366. doi:10.1111/j.1469-8137.1976.tb01471.x
Alston AM (1979) Effects of soil water content and foliar fertilization with nitrogen and phosphorus in late season on the yield and composition of wheat. Aust J Agric Res 30:577–585. doi:10.1071/AR9790577
Aryal B, Neuner G (2010) Leaf wettability decreases along an extreme altitudinal gradient. Oecologia 162:1–9. doi:10.1007/s00442-009-1437-3
Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202:1–8. doi:10.1007/s004250050096
Batten GD (1992) A review of phosphorus efficiency in wheat. Plant Soil 146:163–168
Batten GD, Wardlaw IF, Aston MJ (1986) Growth and distribution of phosphorus in wheat developed under various phosphorus and temperature regimes. Aust J Agric Res 37:459–469. doi:10.1071/AR9860459
Bianchi G, Figini ML (1986) Epicuticular waxes of glaucous and nonglaucous durum wheat lines. J Agric Food Chem 34(3):429–433. doi:10.1021/jf00069a012
Blanco A, Fernández V, Val J (2010) Improving the performance of calcium-containing spray formulations to limit the incidence of bitter pit in apple (Malus x domestica Borkh.). Sci Hortic 127:23–28. doi:10.1016/j.scienta.2010.09.005
Bouma D, Dowling EJ (1976) Relationship between phosphorus status of subterranean clover plants and dry weight responses of detached leaves in solutions with and without phosphate. Aust J Agric Res 27:53–62. doi:10.1071/AR9760053
Brewer CA, Smith WK, Vogelmann TC (1991) Functional interaction between leaf trichomes, leaf wettability and the optical properties of water droplets. Plant Cell Environ 14:955–962. doi:10.1111/j.1365-3040.1991.tb00965.x
Burkhardt J, Basi S, Pariyar S, Hunsche M (2012) Stomatal penetration by aqueous solutions—an update involving leaf surface particles. New Phytol 196:774–787. doi:10.1111/j.1469-8137.2012.04307.x
Domínguez E, Heredia-Guerrero JA, Heredia A (2011) The biophysical design of plant cuticles: an overview. New Phytol 189:938–949. doi:10.1111/j.1469-8137.2010.03553.x
Doroshkov AV, Pshenichnikova TA, Afonnikov DA (2011) Morphological characterization and inheritance of leaf hairiness in wheat (Triticum aestivum L.) as analyzed by computer-aided phenotyping. Russ J Genet 47(6):739–743. doi:10.1134/S1022795411060093
Eichert T, Burkhardt J (2001) Quantification of stomatal uptake of ionic solutes using a new model system. J Exp Bot 52(357):771–781. doi:10.1093/jexbot/52.357.771
Eichert T, Fernández V (2012) Uptake and release of elements by leaves and other aerial plant parts. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Elsevier, London, pp 71–84
Eichert T, Goldbach HE, Burkhardt J (1998) Evidence for the uptake of large anions through stomatal pores. Bot Acta 111:461–466
Eichert T, Kurtz A, Steiner U, Goldbach HE (2008) Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol Plantarum 134:151–160. doi:10.1111/j.1399-3054.2008.01135.x
Ensikat HJ, Ditsche-Kuru P, Neinhuis C, Barthlott W (2011) Superhydrophobicity in perfection: the outstanding properties of the lotus leaf. Beilstein J Nanotechnol 2:152–161. doi:10.3762/bjnano.2.19
Fernández V, Brown PH (2013) From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Front Plant Sci 4:289. doi:10.3389/fpls.2013.00289
Fernández V, Eichert T (2009) Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Crit Rev Plant Sci 28(1):36–68. doi:10.1080/07352680902743069
Fernández V, Del Río V, Abadía J, Abadía A (2006) Foliar iron fertilization of peach (Prunus persica (L.) Batsch): effects of iron compounds, surfactants and other adjuvants. Plant Soil 289:239–252. doi:10.1007/s11104-006-9132-1
Fernández V, Del Río V, Pumariño L, Igartua E, Abadía J, Abadía A (2008a) Foliar fertilization of peach (Prunus persica (L.) Batsch) with different iron formulations: effects on re-greening, iron concentration and mineral composition in treated and untreated leaf surfaces. Sci Hortic 117:241–248. doi:10.1016/j.scienta.2008.05.002
Fernández V, Eichert T, Del Río V, López-Casado G, Heredia JA, Abadía A, Heredia A, Abadía J (2008b) Leaf structural changes associated with iron deficiency chlorosis of field-grown pear and peach—physiological implications. Plant Soil 311:161–172. doi:10.1007/s11104-008-9667-4
Fernández V, Khayet M, Montero-Prado P, Heredia-Guerrero JA, Liakoloulos G, Karabourniotis G, Del Río V, Domínguez E, Tacchini I, Nerín C, Val J, Heredia A (2011) New insights into the properties of pubescent surfaces: peach fruit as model. Plant Physiol 156(4):2098–2108. doi:10.1104/pp. 111.176305
Girma K, Martin KL, Freeman KW, Mosali J, Teal RK, Raun WR, Moges SM, Arnall B (2007) Determination of the optimum rate and growth stage for foliar applied phosphorus in corn. Commun Soil Sci Plant Anal 38:1137–1154. doi:10.1080/00103620701328016
Grammatikopoulos G, Manetas Y (1994) Direct absorption of water by hairy leaves of Phlomis fruticosa and its contribution to drought avoidance. Can J Bot 72(12):1805–1811. doi:10.1139/b94-222
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81:211–224. doi:10.4141/P00-093
Guzmán P, Fernández V, García ML, Khayet M, Fernández A, Gil L (2014) Localization of polysaccharides in isolated and intact cuticles of eucalypt, poplar and pear leaves by enzyme-gold labelling. Plant Physiol Biochem 76:1–6. doi:10.1016/j.plaphy.2013.12.023
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Møller IS, White P (2012) Functions of macronutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Elsevier, London, pp 135–189
Hedley MJ, McLaughlin MJ (2005) Reactions of phosphate fertilizers and by-products in soils. In: Sharpley AN (ed) Phosphorus: agriculture and the environment. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, pp 181–252
Holloway PJ (1969) The effects of superficial wax on leaf wettability. Ann Appl Biol 63:145–153. doi:10.1111/j.1744-7348.1969.tb05475.x
Jeffree CH (2006) The fine structure of the plant cuticle. In: Riederer M, Müller C (eds) Biology of the plant cuticle, annual plant reviews, vol 23. Blackwell, Oxford, pp 11–125
Kannan S (2010) Foliar fertilization for sustainable crop production. Sustain Agric Rev 4:371–402. doi:10.1007/978-90-481-8741-6_13
Khayet M, Fernández V (2012) Estimation of the solubility parameter of model plant surfaces and agrochemicals: a valuable tool for understanding plant surface interactions. Theor Biol Med Model 9:45. doi:10.1186/1742-4682-9-45
Khayet M, Vazquez Alvarez M, Khulbe KC, Matsuura T (2007) Preferential surface segregation of homopolymer and copolymer blend films. Surf Sci 601:885–895. doi:10.1016/j.susc.2006.11.024
Klute A (1986) Water retention: laboratory methods. In: Klute A (ed) Methods of soil analysis, part 1, physical and mineralogical methods, 2nd edn. American Society of Agronomy Inc, Soil Science Society of America Inc, Madison, pp 635–662
Koch K, Barthlott W, Koch S, Hommes A, Wandelt K, Mamdouh W, De-Feyter S, Broekmann P (2006) Structural analysis of wheat wax (Triticum aestivum cv ‘Naturastar’ L.): from the molecular level to three dimensional crystals. Planta 223(2):258–270. doi:10.1007/s00425-005-0081-3
Koontz H, Biddulph O (1957) Factors affecting absorption and translocation of foliar applied phosphorus. Plant Physiol 32:463–470
Malone SR, Mayeux HS, Johnson HB, Polley HW (1993) Stomatal density and aperture length in four plant species grown across a subambient CO2 gradient. Am J Bot 80(12):1413–1418
Martin AE, Reeve R (1955) A rapid manometric method for determination of soil carbonate. Soil Sci 79:187–198
Mason SD, McNeill AM, McLaughlin MJ, Zhang H (2010) Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods. Plant Soil 337:243–258. doi:10.1007/s11104-010-0521-0
Matejovic I (1997) Determination of carbon and nitrogen in samples of various soils by the dry combustion method. Commun Soil Sci Plant Anal 28:1499–1511. doi:10.1080/00103629709369892
McBeath TM, McLaughlin MJ, Kirby JK, Armstrong RD (2012) The effect of soil water status on fertiliser, topsoil and subsoil phosphorus utilisation by wheat. Plant Soil 358(1–2):337–348. doi:10.1007/s11104-012-1177-8
Moody PW (2007) Interpretation of a single-point P buffering index for adjusting critical levels of the Colwell Soil P test. Aust J Soil Res 45:55–62. doi:10.1071/SR06056
Mosali J, Desta K, Teal RK, Freeman KW, Martin KL, Lawles JW, Raun WR (2006) Effect of foliar application of phosphorus on winter wheat grain yield, phosphorus uptake, and use efficiency. J Plant Nutr 29:2147–2163. doi:10.1080/01904160600972811
Noack SR, McBeath TM, McLaughlin MJ (2010) Potential for foliar phosphorus fertilisation of dryland cereal crops: a review. Crop Pasture Sci 61(8):659–669. doi:10.1071/CP10080
Papini A, Tani G, Di Falco P, Brighigna L (2010) The ultrastructure of the development of Tillandsia (Bromeliaceae) trichome. Flora 205(2):94–100. doi:10.1016/j.flora.2009.02.001
Pierce S, Maxwell K, Griffiths H, Winter K (2001) Hydrophobic trichome layers and epicuticular wax powders in Bromeliaceae. Am J Bot 88(8):1371–1389. doi:10.2307/3558444
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press, Melbourne
Riederer M, Friedmann A (2006) Transport of lipophilic non-electrolytes across the cuticle. In: Riederer M, Müller C (eds) Biology of the plant cuticle, annual plant reviews, vol 23. Blackwell, Oxford, pp 250–279
Rodríguez D, Keltjens WG, Goudriaan J (1998) Plant leaf area expansion and assimilate production in wheat (Triticum aestivum L.) growing under low phosphorus conditions. Plant Soil 200:227–240. doi:10.1023/A:1004310217694
Schlegel TK, Schönherr J (2001) Selective permeability of cuticles over stomata and trichomes to calcium chloride. Int Symp Foliar Nutr Perenn Fruit Plants 594:91–96
Schlegel TK, Schönherr J (2002) Stage of development affects penetration of calcium chloride into apple fruits. J Plant Nutr Soil Sci 165:738–745. doi:10.1002/jpln.200290012
Singh D, Singh M (2008) Absorption and translocation of glyphosate with conventional and organosilicone adjuvants. Weed Biol Manag 8:104–111. doi:10.1111/j.1445-6664.2008.00282.x
Singh R, Chadha RK, Verma HN, Singh Y (1977) Response of dryland wheat to phosphorus fertilizer as influenced by profile water storage and rainfall. J Agric Sci (Camb) 88:591–595. doi:10.1017/S0021859600037266
Syers JK, Johnston AE, Curtin D (2008) Efficiency of soil and fertilizer phosphorus use. Reconciling changing concepts of soil phosphorus behaviour with agronomic information. FAO Fertilizer and Plant Nutrition Bulletin 18. Food and Agriculture Organization of the United Nations, Rome
van Oss CJ, Chaudhury MK, Good RJ (1987) Monopolar surfaces. Adv Colloid Interf Sci 28:35–64
van Oss CJ, Chaudhury MK, Good RJ (1988) Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem Rev 88:927–941. doi:10.1021/cr00088a006
Wallihan EF, Embleton TW, Sharpless RG (1964) Response of chlorotic citrus leaves to iron sprays in relation to surfactants and stomatal apertures. Proc Am Soc Hortic Sci 85:210–217
Will S, Eichert T, Fernández V, Möhring J, Müller T, Römheld V (2011) Absorption and mobility of foliar-applied boron in soybean as affected by plant boron status and application as a polyol complex. Plant Soil 344:283–293. doi:10.1007/s11104-011-0746-6
Will S, Eichert T, Fernández V, Müller T, Römheld V (2012) Boron foliar fertilization of soybean and lychee: effects of side of application and formulation adjuvants. J Plant Nutr Soil Sci 175:180–188. doi:10.1002/jpln.201100107
Yu Q, Zhang Y, Liu Y, Shi P (2004) Simulation of the stomatal conductance of winter wheat in response to light, temperature and CO2 changes. Ann Bot 93(4):435–441. doi:10.1093/aob/mch023
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421. doi:10.1111/j.1365-3180.1974.tb01084.x
Acknowledgments
The authors acknowledge funding from the CSIRO Sustainable Agriculture Flagship Fellowship Fund. Victoria Fernández is supported by a “Ramón y Cajal” contract (MINECO, Spain), co-financed by the European Social Fund. Paula Guzmán is supported by a pre-doctoral grant from the Technical University of Madrid. Courtney A. E. Peirce is supported by the Grains Research and Development Corporation of Australia and the Fluid Fertilizer Foundation (USA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philip White.
Rights and permissions
About this article
Cite this article
Fernández, V., Guzmán, P., Peirce, C.A.E. et al. Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus. Plant Soil 384, 7–20 (2014). https://doi.org/10.1007/s11104-014-2052-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2052-6