Abstract
Aims
There is evidence that increased N inputs to boreal forests, via atmospheric deposition or intentional fertilization, may impact negatively on ectomycorrhizal (ECM) fungi leading to a reduced flux of plant-derived carbon (C) back to the atmosphere via ECM. Our aim was to investigate the impact of N fertilization of a Pinus sylvestris (L.) forest stand on the return of recently photoassimilated C via the ECM component of soil respiration.
Methods
We used an in situ, large-scale, 13C-CO2 isotopic pulse labelling approach and monitored the 13C label return using soil gas efflux chambers placed over three different types of soil collar to distinguish between heterotrophic (RH), autotrophic (RA; partitioned further into contributions from ECM hyphae and total RA) and total (RS) soil respiration.
Results
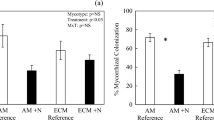
The impact of N fertilization was to significantly reduce RA, particularly respiration via extramatrical ECM hyphae. ECM hyphal flux in control plots showed substantial spatial variability, resulting in mean flux estimates exceeding estimates of total RA, while ECM contributions to RA in N treated plots were estimated at around 30%.
Conclusion
Significant impacts on soil C cycling may be caused by reduced plant C allocation to ECM fungi in response to increased N inputs to boreal forests; ecosystem models so far lack this detail.




Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- ECM:
-
Ectomycorrhiza
- C:
-
Carbon
- CO2 :
-
Carbon dioxide
- CF-IRMS:
-
Continuous-flow isotope ratio mass spectrometer
- N:
-
Nitrogen
- PVC:
-
Polyvinyl chloride
- RS :
-
Soil respiration
- RA :
-
Autotrophic soil respiration
- RH :
-
Heterotrophic soil respiration
References
Ågren GI, Bosatta E, Magill A (2001) Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128:94–98
Bending GD, Read DJ (1995) The structure and function of the vegetative mycelium of ectomycorrhizal plants. VI. Activities of nutrient mobilizing enzymes in birch litter colonised by Paxillus involutus (Fr) Fr. New Phytol 130:411–417
Bhupinderpal-Singh NA, Ottoson-Löfvenius M, Högberg MN, Mellander PE, Högberg P (2003) Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: extending observations beyond the first year. Plant Cell Environ 26:1287–1296
Boberg J, Finlay RD, Stenlid J, Näsholm T, Lindahl BD (2008) Glucose and ammonium additions affect needle decomposition and carbon allocation by the litter degrading fungus Mycena epipterygia. Soil Biol Biochem 40:995–999
Bond-Lamberty B, Wang C, Gower ST (2004) Net primary production and net ecosystem production of a boreal black spruce wildfire chronosequence. Glob Change Biol 10:473–487
Bowling DR, McDowell NG, Bond BJ, Law BE, Ehleringer JR (2002) 13C content of ecosystem respiration is linked to precipitation and vapor pressure deficit. Oecologia 131:113–124
Cannell MGR, Dewar RC (1994) Carbon allocation in trees—a review of concepts for modeling. Adv Ecol Res 25:59–104
Carbone MS, Czimczik CI, McDuffee KE, Trumbore SE (2007) Allocation and residence time of photosynthetic products in a boreal forest using a low-level 14C pulse-chase labeling technique. Glob Change Biol 13:466–477
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Ek H (1997) The influence of nitrogen fertilization on the carbon economy of Paxillus involutus in ectomycorrhizal association with Betula pendula. New Phytol 135:133–142
Ekblad A, Högberg P (2001) Natural abundance of 13C in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia 127:305–308
Franklin O, Högberg P, Ekblad A, Ågren GI (2003) Pine forest floor carbon accumulation in response to N and PK additions: bomb 14C modelling and respiration studies. Ecosystems 6:644–658
Galloway JN, Dentener FJ, Capone DG et al (2004) Nitrogen cycles: past, present, and future. Biogeochem 70:153–226
Heinemeyer A, Hartley IP, Evans SP, De la Fuente JAC, Ineson P (2007) Forest soil CO2 flux: uncovering the contribution and environmental responses of ectomycorrhizas. Glob Change Biol 13:1786–1797
Högberg MN, Högberg P (2002) Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytol 154:791–795
Högberg P, Read DJ (2006) Towards a more plant physiological perspective on soil ecology. Trends Ecol Evol 21:548–554
Högberg P, Nordgren A, Buchmann N et al (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Högberg P, Buchmann N, Read DJ (2006) Comments on Yakov Kuzyakov’s review ‘Sources of CO2 efflux from soil and review of partitioning methods’ [Soil Biology & Biochemistry 38, 425–448]. Soil Biol Biochem 38:2997–2998
Högberg P, Högberg MN, Göttlicher SG et al (2008) High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol 177:220–228
Högberg MN, Briones MJI, Keel SG et al (2010) Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol 187:485–493
Janssens IA, Dieleman W, Luyssaert S et al (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3(5):315–322
Koide RT, Fernandez CW, Peoples MS (2011) Can ectomycorrhizal colonization of Pinus resinosa roots affect their decomposition? New Phytol. doi:10.1111/j.1469-8137.2011.03694.x
Lamarque JF, Kiehl JT, Brasseur GP et al (2005) Assessing future nitrogen deposition and carbon cycle feedback using a multimodel approach: analysis of nitrogen deposition. J Geophys Res Atmos 110, D19303
Langley JA, Hungate BA (2003) Mycorrhizal controls on belowground litter quality. Ecology 84:2302–2312
Langley JA, Chapman SK, Hungate BA (2006) Ectomycorrhizal colonization slows root decomposition: the post-mortem fungal legacy. Ecol Lett 9:955–959
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay RD (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620
Mäkelä A, Valentine HT, Helmisaari HS (2008) Optimal co-allocation of carbon and nitrogen in a forest stand at steady state. New Phytol 180:114–123
Nilsson LO, Wallander H (2003) Production of external mycelium by ectomycorrhizal fungi in a Norway spruce forest was reduced in response to nitrogen fertilization. New Phytol 158:409–416
Olsson P, Linder S, Giesler R, Högberg P (2005) Fertilization of boreal forest reduces both autotrophic and heterotrophic soil respiration. Glob Change Biol 11:1745–1753
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391
Smith SE, Read DL (2008) Mycorrhizal symbiosis. Academic, San Diego
Subke J-A, Inglima I, Cotrufo MF (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a meta-analytical review. Glob Change Biol 12:921–943
Subke J-A, Vallack HW, Magnusson T, Keel SG, Metcalfe DB, Högberg P, Ineson P (2009) Short-term dynamics of abiotic and biotic soil 13CO2 effluxes after in situ 13CO2 pulse labelling of boreal pine forest. New Phytol 183:349–357
Subke J-A, Voke NR, Leronni V, Garnett MH, Ineson P (2011) Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling. J Ecol 99:186–193
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515
Trumbore S (2006) Carbon respired by terrestrial ecosystems—recent progress and challenges. Glob Change Biol 12:141–153
Valentini R, Matteucci G, Dolman AJ et al (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404:861–865
Vitousek PM, Aber JD, Howarth RW et al (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Vogt KA, Publicover DA, Bloomfield J, Perez JM, Vogt DJ, Silver WL (1993) Belowground responses as indicators of environmental change. Environ Exp Bot 33:189–205
Wallander H, Nylund JE (1992) Effects of excess nitrogen and phosphorus starvation on the extramatrical mycelium of ectomycorrhizas of Pinus sylvestris L. New Phytol 120:495–503
Wallander H, Nilsson LO, Hagerberg D, Baath E (2001) Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. New Phytol 151:753–760
Wallander H, Ekblad A, Bergh J (2011) Growth and carbon sequestration by ectomycorrhizal fungi in intensively fertilized Norway spruce forests. Forest Ecol Manag 262:999–1007
Wallenda T, Kottke I (1998) Nitrogen deposition and ectomycorrhizas. New Phytol 139:169–187
Wallenda T, Schaeffer C, Einig W et al (1996) Effects of varied soil nitrogen supply on Norway spruce (Picea abies [L] Karst). 2. Carbon metabolism in needles and mycorrhizal roots. Plant Soil 186:361–369
White A, Cannell MGR, Friend AD (2000) The high-latitude terrestrial carbon sink: a model analysis. Glob Change Biol 6:227–245
Acknowledgements
This study was supported by grants from SLU, the Kempe Foundations, the research councils VR and FORMAS (to P.H.) and the UK Natural Environment Research Council (NERC grant ref.NE ⁄ E004512 ⁄ 1). We are grateful to Sylvia Toet (York) for loan of the CO2 flux chamber as well as to Toby Gass, Erica Nocchi, Sonja Keel and other members of the CANIFLEX team for assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric Paterson.
Rights and permissions
About this article
Cite this article
Vallack, H.W., Leronni, V., Metcalfe, D.B. et al. Application of nitrogen fertilizer to a boreal pine forest has a negative impact on the respiration of ectomycorrhizal hyphae. Plant Soil 352, 405–417 (2012). https://doi.org/10.1007/s11104-011-1005-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1005-6